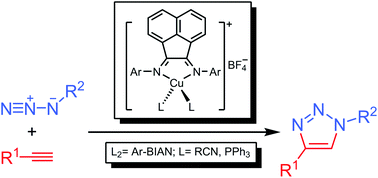
A series of Ar-BIAN-based copper(I) complexes (where Ar-BIAN = bis(aryl)acenaphthenequinonediimine) were synthesised and characterised by 1H and 13C NMR spectroscopies, FT-IR spectroscopy, MALDI-TOF-MS spectrometry, cyclic voltammetry and single crystal X-ray diffraction. The bis-chelated complexes of general formula [Cu(Ar-BIAN)2]BF4 (where Ar = C6H5 (1), 4-iPrC6H4 (3), 2-iPrC6H4 (4)) were prepared by reaction of [Cu(NCMe)4]BF4 with two equivalents of the corresponding Ar-BIAN ligands, in dichloromethane, while the mono-chelated complexes of the type [Cu(Ar-BIAN)L2]BF4 (where Ar = 2,6-iPr2C6H3, L = PhCN (6); Ar = 4-iPrC6H4, L = PPh3 (7)) were readily accessible by treatment of [Cu(NCR)4]BF4 (R = Me, Ph) with one equivalent of the corresponding Ar-BIAN ligands in the absence or presence of two equivalents of PPh3, in the same solvent. The structures of complexes 3, 4, 6 and 7 were obtained by single crystal X-ray diffraction, showing distorted tetrahedral geometries around the copper centres in all cases. The electrochemical studies of these complexes and of the already reported [Cu(2,4,6-Me3C6H2-BIAN)2]BF4 (2) and [Cu(2,6-iPr2C6H3-BIAN)(NCMe)2] (5), demonstrated that the bis-chelated complexes 1–4 undergo a reversible one-electron reduction or oxidation processes on copper, while the mono-chelated complexes 5–7 show a partially reversible oxidation and an irreversible reduction feature. Both kinds of (Ar-BIAN)copper(I) complexes are active catalysts for the copper(I)-catalysed azide–alkyne cycloaddition reaction (CuAAC). Complex 7, bearing PPh3 ligands, exhibits the highest catalytic activity, which is comparable with that of the typical CuSO4–sodium ascorbate catalyst system.

 Please wait while we load your content...
Please wait while we load your content...