Epoxidation of olefins with homogeneous catalysts – quo vadis?
Simone A.
Hauser
,
Mirza
Cokoja
* and
Fritz E.
Kühn
*
Chair of Inorganic Chemistry/Molecular Catalysis, Catalysis Research Center, Technische Universität München, Ernst-Otto-Fischer-Straße 1, D-85747 Garching, Germany. E-mail: fritz.kuehn@ch.tum.de; mirza.cokoja@ch.tum.de; Fax: +49 89 289 13473
First published on 24th October 2012
Abstract
The epoxidation of olefins catalyzed by molecular transition metal compounds is a research field, which has been extensively studied over the past forty years. To date, numerous types of complexes have been presented as widely applicable and highly efficient. This Perspective Article gives a summary of the most active catalysts for the epoxidation of some most frequently applied olefins, such as cyclooctene, 1-octene, prochiral olefins and industrially relevant olefins.
 Simone A. Hauser | Simone A. Hauser was born in Switzerland in 1985. In 2009 she graduated in molecular and biological chemistry at the Ecole Polytechnique Fédérale de Lausanne (Switzerland) under the supervision of Professor Paul J. Dyson. In the same year, she moved to the Technische Universität München (Germany) to join the group of Professor Fritz E. Kühn, working as a PhD candidate on olefin epoxidation catalyzed by organorhenium and organomolybdenum complexes. |
 Mirza Cokoja | Mirza Cokoja studied Chemistry at the Ruhr-University Bochum, Germany. He received his PhD under the guidance of Roland A. Fischer in 2007. In 2008, he joined the team of Bruno Chaudret at the CNRS Laboratoire de Chimie de Coordination in Toulouse, France as a postdoc. Since 2009, he has been a research group leader at the Chair of Inorganic Chemistry of the TU München. His research interests are the activation of small molecules with organometallic catalysts, oxidation catalysis and the synthesis of 2- and 3-dimensional metal–organic building blocks for the design of porous coordination polymers. |
 Fritz E. Kühn | Fritz E. Kühn studied chemistry at the TU München, where he received his PhD with W. A. Herrmann in 1994. After postdoctoral research with F. A. Cotton (Texas A&M University, USA) 1995/96 he finished his Habilitation in Munich to become “Privatdozent” in 2000. From June 2005 to March 2006 he was Deputy Chair of Inorganic Chemistry at TUM. In April 2006 he was appointed Principal Researcher at the Instituto Técnológico e Nuclear (ITN) in Sacavém, Portugal. In December 2006 he returned to TUM as Professor of Molecular Catalysis and since October 2007 he has also been acting Chair of Inorganic Chemistry. F. E. Kühn is author of ca. 300 scientific publications. |
Introduction
The epoxidation of olefins is a reaction of high relevance in both industry and academia. Epoxides are very important intermediates in the chemical industry, particularly for the synthesis of various polymers (polyglycols, polyamides, polyurethanes, etc.),1 but they are also being used in the synthesis of fine chemicals, such as pharmaceuticals, food additives, or flavor and fragrance compounds.2 The biggest market is for propylene oxide, which is currently produced on a scale of 8 million tons per year with an expected annual increase of 5%.3 For ethylene and propylene oxide, heterogeneous catalysts, such as Ag@Al2O3 (for ethylene oxide) and titania-doped zeolite-type silicates (TS-1, for propylene oxide), developed by EniChem, Evonik, Dow and BASF are the state-of-the-art processes.3 The main reasons for their application are the catalyst recycling, which is intrinsically easier for heterogeneous catalysts, as well as their long-time stability, product selectivity and the type of oxidant. Whereas the heterogeneous catalysts usually rely on cheap oxygen, either used directly (→ ethylene oxide) or indirectly (e.g. for the production of H2O2 or organic peroxides), homogeneous catalysts often require rather ‘exotic’ (from an industrial perspective) oxidants, such as NaOCl, iodosobenzene, amine- or pyridine-N-oxides. Thus, molecular epoxidation catalysts, such as the most prominent examples by Katsuki–Sharpless,4 Kochi–Jacobsen,5 Herrmann6 and others, have so far mainly been used in the synthesis of more or less sophisticated organic molecules, as described in several reviews.7,8 Asymmetric epoxidation of prochiral olefins is, of course, difficult to achieve with heterogeneous catalysts, and molecular catalysts are considered to be much more promising, allowing for tuning the organic ligands at the metal, giving high enantiomeric excesses (ee). For these reactions, especially salen-type ligands appear to be the best choice;9,10 they, however, render the catalysts very expensive. Thus, a lot of effort has been devoted to the development of other molecular catalysts, such as dioxomolybdenum complexes and half-sandwich cyclopentadienyl molybdenum compounds.11–13 In the case of the very versatile catalyst methyltrioxorhenium (MTO), which utilizes aqueous H2O2 as an oxidant, all attempts to create a chiral version leading to high enantioselectivity in epoxidation have so far failed.14,15 Transfer of chirality can be obtained with lanthanide–1,1′-binaphthyl-2,2′-diol (BINOL) catalytic systems. They are, however, primarily suited for enones.16,17 Meanwhile, metal-free organocatalysts are also used for asymmetric epoxidation of complex organic olefins, and they are successfully applied with a variety of oxidants: oxone (KHSO5), aqueous hydrogen peroxide (H2O2), urea hydrogen peroxide (UHP) and even molecular oxygen (O2).18–20 Nevertheless, these catalyst systems are usually applied for special organic substrates, where the epoxidation is normally one of many reaction steps towards a particular chemical. Many reports on catalytic epoxidation in a homogeneous phase notoriously do not comment on factors, which are important for an efficient, long-term (industrial) application, such as turn-over frequencies (TOF) and -numbers (TON), catalyst/product separation and recycling, and catalyst stability. Further, the best known molecular epoxidation catalysts were not tested for ethylene and propylene epoxidation. In order to achieve an efficient catalyst separation, many groups focused on the immobilization on solid supports,21 which is, however, often associated with a significant loss of catalytic activity. Another extensively studied possibility are two-phase reactions in ionic liquid (IL) media, where the catalyst is dissolved in the IL and substrate and product are immiscible.22,23Looking at the recent developments in epoxidation with molecular metal catalysts, the question can be raised, what are the perspectives, i.e. the demands for an epoxidation catalyst? Hence, the motivation to write this Perspective Article was driven by two questions:
(1) Which are the state-of-the-art catalysts for different types of olefins, such as cyclooctene, 1-octene, styrene, prochiral olefins, but also ethylene and propylene? To date, complexes of various metals bearing different ligands are known. Which of all those complexes is – among the plethora of available catalysts – the most widely applicable?
(2) Do these catalysts exhibit a perspective to be used in industrial, large-scale epoxidation reactions, going well beyond being used for one of several (or many) synthesis steps in fine chemical synthesis? Do they exhibit a perspective for the epoxidation of ethylene and propylene; can they compete to the well-established industrial processes?
Epoxidation of cis-cyclooctene
cis-Cyclooctene is the most used test substrate in academic research. This olefin is particularly suitable for assessing the catalytic performance of a new compound, as it is easily epoxidized in the presence of a catalyst. Hence, it is broadly used as a benchmark substrate for catalyst comparison. However, the reaction conditions (temperature, catalyst concentration, duration, etc.) strongly influence the catalyst activity. Thus, it is of great importance to select a suitable reaction system for every catalyst and to bear in mind the conditions for the comparison of the catalyst performances. A specific characteristic exists that is intended to enable easy comparison, namely the turn-over frequency (TOF), which determines the amount of substrate (in mol) converted by the applied amount of catalyst (in mol) per unit time (usually given in hours). This number is typically calculated during the initial phase of the catalytic reaction, where the kinetics curve of product build-up shows a steep increase, the concentration of substrate molecules is still high and diffusion is not a limiting factor. Unfortunately, the TOF is often not mentioned/determined in publications, so other features have to be considered: the faster a quantitative conversion is achieved, the better the catalyst appears to work. Another possibility of differentiation is the turn-over number (TON), which states the catalyst lifetime. It is calculated by the maximum amount of substrate (in mol) converted by the applied amount of catalyst (in mol) during its lifetime. Unfortunately, the published TONs are often calculated based on one single catalytic run, ignoring the remaining catalyst activity and lifetime.There are three current benchmark catalysts (Fig. 1), all of them showing very high TOFs. There are two molybdenum-based complexes: [Mo2(OtBu)6] (1) reaches a TOF of above 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, i.e. 25
000 h−1, i.e. 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 per Mo center, at a catalyst loading of 0.05 mol% with nearly quantitative yield.24 The so-called ansa-complex [Mo(η5-C5H4(CH(CH2)3)-η1-CH)(CO)3] (2) even reaches a TOF of 44
000 h−1 per Mo center, at a catalyst loading of 0.05 mol% with nearly quantitative yield.24 The so-called ansa-complex [Mo(η5-C5H4(CH(CH2)3)-η1-CH)(CO)3] (2) even reaches a TOF of 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 (at a concentration of 0.05 mol% in a room-temperature ionic liquid (RTIL)).25 A TOF of almost 40
000 h−1 (at a concentration of 0.05 mol% in a room-temperature ionic liquid (RTIL)).25 A TOF of almost 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 has been attained with 0.01 mol% methyltrioxorhenium (MTO, 3), applied with an excess of pyrazole in hexafluoroisopropanol, and yielding full conversion of the substrate.26 Further (pre)-catalysts exhibit a TOF of above 4000 h−1 (Table 1, entries 4–8). Interestingly, these are all molybdenum-based complexes, working with tert-butylhydroperoxide (TBHP) in decane as an oxidant.27–31 The dioxomolybdenum catalyst 9 (Table 1, entry 9) has a high catalytic activity,32 as well as the tungsten-based complexes 10 and 11 (that are applied with aqueous H2O2 as an oxidant; Table 1, entries 10 and 11). At low catalyst loadings, they reach quantitative substrate conversion within a reasonable time.33,34 Peracetic acid is used in the reaction with the dimeric Mn-catalyst 12, which quantitatively converts cyclooctene to the epoxide within 3 min (at 3 mol% catalyst loading, entry 12).35
000 h−1 has been attained with 0.01 mol% methyltrioxorhenium (MTO, 3), applied with an excess of pyrazole in hexafluoroisopropanol, and yielding full conversion of the substrate.26 Further (pre)-catalysts exhibit a TOF of above 4000 h−1 (Table 1, entries 4–8). Interestingly, these are all molybdenum-based complexes, working with tert-butylhydroperoxide (TBHP) in decane as an oxidant.27–31 The dioxomolybdenum catalyst 9 (Table 1, entry 9) has a high catalytic activity,32 as well as the tungsten-based complexes 10 and 11 (that are applied with aqueous H2O2 as an oxidant; Table 1, entries 10 and 11). At low catalyst loadings, they reach quantitative substrate conversion within a reasonable time.33,34 Peracetic acid is used in the reaction with the dimeric Mn-catalyst 12, which quantitatively converts cyclooctene to the epoxide within 3 min (at 3 mol% catalyst loading, entry 12).35
 | ||
| Fig. 1 Three outstanding (pre-)catalysts for the epoxidation of cyclooctene. | ||
| Entry | Complex | T/°C | Time/h | Solvent | Catalyst conc. (mol%) | Yield (%) | TOF/h−1 | Ref. |
|---|---|---|---|---|---|---|---|---|
| a L1 = 2-(2′-hydroxyphenyl)oxazolinate; L2 = 5,5′-bis-methoxycarbonyl-2,2′-bipyridine; L3 = 5-(2′-hydroxypheny1)pyrazole; L4 = 2[(2-hydroxy-2-phenylethylimino)methyl]phenol; L5 = tetrahexylammonium; L6 = tetrabutylammonium; L7 = E-1,2-bis(2,2′-bipyridyl-6-yl)ethene. b Cpox = cyclopentadienyl ligand with a pendant oxazoline group. c Not stated in the article. | ||||||||
| 1 | [Mo2(OtBu)6] (1) | 25 | 0.03 | CH2Cl2 | 0.05 | 86 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
24 |
| 2 | [Mo(η5-C5H4(CH(CH2)3)-(η1-CH))(CO)3] (2) | 25 | 0.03 | Ionic liquid | 0.05 | —c | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
25 |
| 3 | [CH3ReO3] (3) | 0 | 3 | Hexafluoroisopropanol | 0.01 | 100 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
26 |
| 4 | [Mo(O)2(L1)2]a (4) | 80 | 1 | 1,2-Dichloroethane | 0.02 | 96 | 4800 | 27 |
| 5 | [Mo(O)2(Cl)2(L2)]a (5) | 25 | 24 | Ionic liquid | 0.1 | 99 | 8090 | 28 |
| 6 | [CpoxMo(CO)2(NCMe)]BF4b (6) | 55 | 0.5 | CHCl3 | 0.2 | 90 | 5421 | 29 |
| 7 | [Mo(η5-C5(C6H5)5)(O)2Cl] (7) | 55 | 24 | — | 0.1 | 80 | 4000 | 30 |
| 8 | [Mo(O)2(L3)2]a (8) | 55 | 4 | CHCl3 | 0.02 | >99 | 5000 | 31 |
| 9 | [Mo(O)2(L4)(CH3OH)]a (9) | 80 | 0.75 | 1,2-Dichloroethane | 1 | 100 | nd | 32 |
| 10 | (L5)2[W2(O)3(O2)4] (10) | 80 | 4 | MeCN | 0.2 | 100 | nd | 33 |
| 11 | (L6)4[γ-SiW10O34(H2O)2] (11) | 32 | 2 | MeCN | 0.8 | >99 | nd | 34 |
| 12 | [Mn2L7(μ-OAc)3]PF6 (12) | 25 | 0.05 | MeCN | 3 | 100 | nd | 35 |
More demanding substrates
After the catalyst performance has been assessed in the epoxidation of cyclooctene, a range of aromatic or aliphatic substrates are available to show the catalyst potential. There are two model substrates, an aliphatic terminal olefin (1-octene) and an aromatic alkene (styrene). Both are more difficult to epoxidize and are prone to ring-opening byproduct formation, thus allowing a more subtle distinction of the catalytic power of a catalyst.1-Octene
MTO (3) confirms its great potential as an epoxidation catalyst, as it shows the best performance in the epoxidation of 1-octene. Applied under similar reaction conditions as for cyclooctene, the catalytic system reaches a TOF of nearly 5000 h−1, yielding 89% epoxide within 3 h.26Together with compound 1 (86% yield in 16 h with 0.5 mol% catalyst), two other Mo-complexes presented in the previous section exhibit high catalytic activities for terminal olefins as well (Fig. 2).32,35 Bhattacharyya et al. reported the successful application of an ionic diperoxo tungsten complex, PPh4[WO(O2)2(1-(2′-hydroxy-phenyl)ethanonoxime)] (13), in the epoxidation of 1-octene with H2O2.36 A very low catalyst concentration (0.05 mol%) yields 95% 1-octene oxide within 2.25 h in acetonitrile at 40 °C. The polyoxovanadotungstate complex [μ-1,2-H2SiV2W10O40]4− (14) is also a good catalyst for terminal olefins.37 With H2O2 as an oxidant, this system reaches a yield of 93% (with 99% selectivity) at a concentration of 5 mol% at room temperature.
 | ||
| Fig. 2 Efficient catalysts for the epoxidation of cyclooctene as well as 1-octene. | ||
Styrene
One of the catalysts succeeding in high yielding epoxidation of styrene is again MTO (3). It reaches quantitative substrate conversion in 3 h at 25 C, with only 0.5 mol% catalyst applied.38 A very high TOF (>3000 h−1) has been obtained with a manganese complex containing a tetradentate nitrogen base ligand (15).39 This complex, prepared by Costas et al., yields 86% styrene oxide within 3 min with peracetic acid as an oxidant. Comba and co-workers optimized the reaction conditions for iron bispidine complexes,40 achieving with one of them (16) nearly quantitative yields at room temperature within 24 h (with iodosobenzene as an oxidant). Good results have also been obtained with the molybdenum complex PPh4[MoO(O2)2(1-(2′-hydroxyphenyl)ethanonoxime)] (17)36 as well as with the simple iron trichloride hexahydrate (18).41 In the presence of pyridine-2,6-dicarboxylic acid and H2O2, 18 forms an efficient catalytic system that yields 91% epoxide at a catalyst loading of 5 mol%, within 1 h at room temperature.Terpenes
Mono- and bicyclic monoterpene hydrocarbons occur in many essential oils and their byproducts, and they represent an important group of renewables which can be catalytically converted to fine chemicals. The industrially most important member of the terpene family is 1,2-limonene oxide. It finds application in the perfume industry and is widely used as a building block in the manufacture of a range of important commercial products.42,43 α-Pinene is also utilized as a starting material in the synthetic manufacture of flavors and fragrances.42,44 Moreover, it is an intermediary species in the synthesis of Taxol, an anticancer drug.45 The bicyclic terpene camphene is found in a variety of living conifers as well as in fossilized amber.46 It is a valuable precursor for complex molecules, e.g. in the synthesis of a spiro-ring system containing a benzopyram moiety.47 Another member of the terpene family is the terpenoid geraniol. This prochiral allylic alcohol is commonly used in perfumery, due to its own fresh, citrus flavor, and as a precursor in the synthesis of a variety of other floral fragrances.48The following paragraphs assemble the best reaction protocols for terpene oxidation (substrates are depicted in Fig. 3), based on substrate conversion and epoxide selectivity, from which the latter is by far more important.
 | ||
| Fig. 3 Terpene substrates covered in this article: (a) limonene, (b) α-pinene, (c) camphene, and (d) geraniol. | ||
Limonene
Quantitative and selective conversion of limonene to 1,2-limonene oxide has only been reported in three publications, and an elaborate substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidant
oxidant![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio has proven to be crucial. Upon mixing limonene, MTO (3; 5 mol%) and UHP (200 mol%) in chloroform, Boehlow and Spilling claimed 99% yield of the monoepoxide after 30 min at 20 °C.49 The other examples reaching these results involve molybdenum-based catalysts: Drieß and co-workers applied the pre-catalyst [Mo2(OtBu)6] (1), together with TBHP as an oxidant, and received >99% yield in 40 min.24 This reaction has been carried out at room temperature, which is advantageous compared to the third catalytic system (55 °C), developed by Royo et al.29 The complexes [CpoxMo(CO)2(NCMe)]BF4 (6) and [CpoxMo(C3H5)(CO)2] (19) perform equally well: within 1 h, the conversion of limonene to 1,2-limonene oxide is complete (with TBHP as an oxidant). A nearly quantitative conversion with very good epoxide selectivity (98%) has been achieved as well with a 1 mol% tungsten-based complex, [(C6H13)4N]3[PO4W2(O)2(μ-O2)2(O2)22] (20), and H2O2 as an oxidant at room temperature in only 30 min.50 Manganese complexes, e.g. Jacobsen's catalyst51 (21) and Mn(OAc)3·2H2O (22),52 also proved to be suitable catalysts for this reaction, showing excellent selectivity to 1,2-limonene oxide (100%) with acceptable conversion (55% and 96%, respectively). Kühn et al. published a detailed investigation on the best reaction conditions employing MTO (3) as a catalyst.53 The highest selectivity towards 1,2-limonene oxide is obtained with a ratio of limonene
catalyst ratio has proven to be crucial. Upon mixing limonene, MTO (3; 5 mol%) and UHP (200 mol%) in chloroform, Boehlow and Spilling claimed 99% yield of the monoepoxide after 30 min at 20 °C.49 The other examples reaching these results involve molybdenum-based catalysts: Drieß and co-workers applied the pre-catalyst [Mo2(OtBu)6] (1), together with TBHP as an oxidant, and received >99% yield in 40 min.24 This reaction has been carried out at room temperature, which is advantageous compared to the third catalytic system (55 °C), developed by Royo et al.29 The complexes [CpoxMo(CO)2(NCMe)]BF4 (6) and [CpoxMo(C3H5)(CO)2] (19) perform equally well: within 1 h, the conversion of limonene to 1,2-limonene oxide is complete (with TBHP as an oxidant). A nearly quantitative conversion with very good epoxide selectivity (98%) has been achieved as well with a 1 mol% tungsten-based complex, [(C6H13)4N]3[PO4W2(O)2(μ-O2)2(O2)22] (20), and H2O2 as an oxidant at room temperature in only 30 min.50 Manganese complexes, e.g. Jacobsen's catalyst51 (21) and Mn(OAc)3·2H2O (22),52 also proved to be suitable catalysts for this reaction, showing excellent selectivity to 1,2-limonene oxide (100%) with acceptable conversion (55% and 96%, respectively). Kühn et al. published a detailed investigation on the best reaction conditions employing MTO (3) as a catalyst.53 The highest selectivity towards 1,2-limonene oxide is obtained with a ratio of limonene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTO
MTO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t-butylpyridine
t-butylpyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 of 100
H2O2 of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150, leading to the formation of 1,2-limonene oxide with a yield of 77% (96% selectivity with respect to substrate conversion) after 1 h (25 °C, CH2Cl2 as the solvent).
150, leading to the formation of 1,2-limonene oxide with a yield of 77% (96% selectivity with respect to substrate conversion) after 1 h (25 °C, CH2Cl2 as the solvent).
α-Pinene
For the epoxidation of α-pinene, MTO (3) is again the catalyst of choice, as the results show in Table 2. The best and straightforwardly applicable system was reported only last year by Kühn and co-workers (Table 2, entry 4).54Camphene
Camphene oxide can be successfully obtained through epoxidation of camphene with a variety of transition metal complexes, in contrast to α-pinene. Nevertheless, MTO (3) features once again among the best catalysts.58 In the biphasic system H2O2–CH2Cl2 at 25 °C, it leads to 97% yield of camphene oxide (with a selectivity of 98%). Another potent catalytic system was reported by Mirkhani et al.59 A Mn(salen)OAc complex (23) effectively catalyzes the epoxidation of camphene with sodium periodate (NaIO4), reaching 97% yield in 10 min at 25 °C. A far less exotic oxidant, namely molecular oxygen at atmospheric pressure, needs the tris(tetrazolylenolate)iron(III) complex (24) to catalyze the epoxidation of camphene.60 1 mol% catalyst is sufficient to yield 87% epoxide after 4 h at 20 °C. The same oxidant (O2) is used with the cobalt-based complex 25 (Fig. 4), leading to a good yield (72%) of camphene oxide.61 | ||
| Fig. 4 A good catalyst for camphene epoxidation with O2 as the oxidant. | ||
Geraniol
The first protocol for catalytic asymmetric epoxidation was reported by Katsuki and Sharpless in 1980.4 The components of the catalytic system, i.e. (+)- or (−)-diethyl tartrate, titanium tetraisopropoxide (26) and TBHP, are all commercially available at moderate cost (Scheme 1). This fact, as well as the simplicity of the system and the high selectivity, led to the Nobel Prize for K. B. Sharpless in 2001.62 Although the substrate scope of the system is limited to allylic alcohols, it is still among the best ones for the epoxidation of geraniol: reaching a yield of 77% (after 18 h at −20 °C), the enantiomeric excess of 95% has not often been surpassed until now. After optimization of this system by addition of molecular sieves and reduction of the catalyst loading to 5 mol%, Sharpless et al. managed to obtain a higher yield of 2,3-epoxy-geraniol (99%), but the ee slightly decreased (91%).63 The same ee (91%) is obtained with [Ti(OiPr)4] (26) in the presence of L-diisopropyl tartrate (L-DIPT), however, the yield is somewhat lower (58%).64 The advantage of this catalytic system consists in the possibility to apply a renewable oxidant, namely tertiary furyl hydroperoxide. If the epoxidation of geraniol is catalyzed by a [VO(OiPr)3]–chiral bishydroxamic acid system (27) with aqueous TBHP as an oxidant, a yield of 68% and an ee of 95% are reached, by applying only 1 mol% catalyst.65 | ||
| Scheme 1 Sharpless epoxidation of geraniol. | ||
Prochiral olefins
Apart from terpenes and terpenoids, a variety of prochiral olefins are ubiquitous in fine chemical industry. The beneficial characteristics of epoxidation reactions is the ability of creating two stereogenic centers with one chemical reaction, and the epoxides are highly versatile chiral building blocks in pharmaceuticals manufacture and the production of flavors and fragrances. In this review, a selection of the state-of-the-art catalysts for the homogeneous asymmetric epoxidation of prochiral olefins is presented. They have been chosen in terms of enantioselectivity and activity, all by accepting higher catalyst loadings in favour of shorter reaction times, as with time, byproducts accumulate. Solely catalytic systems that effectively induce enantioselective products are presented herein.Aryl-substituted olefins
A wide range of transition-metal based catalysts have been applied in the epoxidation of trans-β-methylstyrene, however, only a handful of ruthenium complexes, which are summarised in Table 3, reach high yields combined with very good ee's.As in the case of trans-β-methylstyrene, epoxidation of stilbene often leads to racemic mixtures (Fig. 5). The above-mentioned Ru-based catalyst 31 also performs well in the epoxidation of trans-stilbene, giving the best yield (100%) with a moderate ee of 54%.69 Slightly higher selectivity to the S,S-epoxide shows [Ru(pydic)(pyboxip)] (32; 80% yield, 63% ee), although the reaction time (96 h) is significantly longer.70 A very promising catalyst system has been developed by Beller et al., consisting of FeCl3·6H2O (18), pyridine-2,6-dicarboxylic acid, and a readily accessible chiral N-arenesulfonyl-N′-benzyl-substituted ethylenediamine ligand.71 Stirring of the reaction mixture (H2O2 as an oxidant) for 1 h at room temperature yields 87% R,R-stilbene oxide with an ee of 42%.
 | ||
| Fig. 5 Selected aryl-substituted olefins that are discussed in this article: (a) trans-β-methylstyrene and (b) trans-stilbene. | ||
Cyclic alkenes
For the cyclic prochiral alkenes, the class of benzene-fused cycloalkenes has been chosen (Scheme 2). 1,2-Dihydronaphthalene (n = 2) is the most often applied substrate. Various catalytic systems (Table 4, entries 1, 4, 7, 9, 10) achieve high ee's in combination with good yields. Three of the catalysts showing an excellent performance in the epoxidation of 1,2-dihydronaphthalene can also be successfully applied in the synthesis of indene oxide (Table 4, entries 2, 5, 8). The reaction times are mostly somewhat longer, however, the selectivity is kept at a high level (>97%). The enantioselective epoxidation of benzocycloheptene is even less prone to be used as assessment for the catalyst performance. The entries 3, 6, and 11 in Table 4 present the only catalysts that were also applied in the epoxidation of this olefin. | ||
| Scheme 2 Epoxidation of benzene-fused cycloalkenes. | ||
| Entry | Catalyst | Substrate | T/°C | Time/h | Solvent | Oxidant | Yield (%) | ee (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a 1,2-Dihydronaphthaline. b Indene. c Benzocycloheptene. d Tritylhydroperoxide. e Triphenylphosphine oxide-H2O2/maleic anhydride. | |||||||||
| 1 |

|
n = 2a | 25 | 12 | CH2Cl2 | H2O2 | >99 | >99 | 72 |
| 2 | n = 1b | 24 | Ethyl acetate | 87 | 99 | ||||
| 3 | n = 3c | 24 | 85 | 98 | |||||
| 4 |

|
n = 2a | 40 | 6 | CH2Cl2–phosphate buffer | H2O2 | 99 | 98 | 73 |
| 5 | n = 1b | 6 | 98 | 98 | |||||
| 6 | n = 3c | 9 | 77 | 97 | |||||
| 7 |

|
n = 2a | 25 | 3 | CH2Cl2 | H2O2 | 90 | 95 | 74 |
| 8 | n = 1b | 18 | 88 | 97 | |||||
| 9 |

|
n = 2a | 25 | 18 | CH2Cl2 | THPd | 98 | 95 | 75 |
| 10 |

|
n = 2a | −18 | 1 | CH2Cl2–DMF | UHP | 70 | 73 | 76 |
| 11 | n = 3c | 1.5 | POHP/MAe | 73 | 92 | ||||
α,β-Unsaturated ketones
The asymmetric epoxidation of electron-deficient olefins is especially useful in the synthesis of a variety of natural products and pharmaceuticals. We have chosen chalcone as a representative example for the determination of the most potent catalysts (Scheme 3). Interestingly, lanthanoid complexes give better results than the catalysts presented in the previous paragraphs. Shibasaki's report on Ln–BINOL catalytic systems17 marked the beginning of extensive research to find the optimum reaction conditions. Table 5 summarizes the best catalytic systems, specifying the components present in the reaction mixture in addition to the chiral ligand BINOL. The systems are listed with their single components, as the real catalytic species is still a matter of investigation. | ||
| Scheme 3 Enantioselective epoxidation of the α,β-unsaturated ketone chalcone. | ||
| Entry | Metal precursor | Catalyst conc. (mol%) | T/°C | Time/h | Solvent | Additive | Oxidant | Yield (%) | ee (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| a Cumene hydroperoxide. | ||||||||||
| 1 | Yb(OiPr)3 (38) | 5 | 25 | 1 | THF | H2O | TBHP | 99 | 81 | 77 |
| 2 | La(OiPr)3 (39) | 5 | 25 | 0.5 | THF | Ph3PO | TBHP | 99 | 96 | 78 |
| 3 | La(OiPr)3 (39) | 5 | 25 | 0.25 | THF | Ph3AsO | TBHP | 98 | 96 | 79 |
| 4 | La(OiPr)3 (39) | 5 | 25 | 0.2 | THF | Ph3PO | CHPa | 99 | >99 | 80 |
| 5 | ZnEt2 (40) | 20 | 25 | 1 | Et2O | — | CHPa | 99 | 90 | 81 |
Epoxidation of industrially relevant bulk olefins
Ethylene
Nowadays, industrial ethylene oxide production mainly employs the heterogeneous oxidation of ethylene by O2 with a silver-based catalyst. This reaction was developed by Shell in 1958,82 and still presents the method of choice. Nevertheless, there is some need for further research: first, the reaction conditions of the heterogeneous process are quite harsh, high temperatures (above 200 °C) have to be maintained, and secondly, the gas phase, an ethylene oxide–O2 mixture, represents a security risk, as it is a potential explosive. Thirdly, it is desirable to reduce the large amount of CO2 emitted as a byproduct (through combustion of ethylene and ethylene oxide). These requisites for a ‘greener’ method are all met by a recent publication by Subramaniam et al.83 To the best of our knowledge, it represents the only report on the homogeneous transition-metal catalyzed epoxidation of ethylene. The procedure consists of a constant-pressure batch reactor, which is charged with an alcoholic solvent (preferably methanol), the catalyst and oxidant (MTO (3) and H2O2) as well as pyridine-N-oxide as Lewis base. The reactor is pressurized with 50 bar ethylene and heated to 40 °C. With this method, ethylene oxide yields of over 50% after 9 h reaction time have been achieved.83Propylene
Propylene oxide is one of the major commodity chemicals used in chemical industry, and its production is mainly based on three different processes. The benchmark is the aforementioned hydrogen peroxide–propylene oxide (HPPO) process using the TS-1 (titania-doped zeolite-type silicates) catalyst. The non-catalytic chlorohydrin method (water as an oxygen source) is outfashioned, as large amounts of Cl2 are consumed and a lot of chlorinated toxic waste is produced. The hydroperoxide method, which is also known as the Halcon–ARCO process (tert-butyl hydroperoxide), and further developed by the Sumitomo company (cumene hydroperoxide) is also currently used.3,84–86All processes require in situ production of the oxidants which is usually a quite energy-demanding process, and the direct oxidation by O2 is prohibited, due to side reactions and combustion, respectively. On the other hand, the catalytic processes suffer from a decomposition of the oxidant, particularly in the case of TS-1. Therefore, the direct oxidation of propylene to propylene oxide, in a more environmentally friendly way and with a minimal formation of byproducts, has been one of the most desirable goals for the chemical industry. Homogeneous catalytic epoxidation of propylene suffers from the difficulty of catalyst separation and re-use; nevertheless, the reaction can take place under milder conditions. The first report of homogeneous catalytic propylene epoxidation with high selectivity to propylene oxide dates back to 1995. An EuCl3 catalytic system (41, in the presence of Zn powder and acetic acid) allows the selective synthesis of propylene oxide with molecular oxygen as an oxidant, however, the yields have been very low (2%).87 Zuwei et al. managed to circumvent the drawbacks of homogeneous catalysis by developing a reaction-controlled phase-transfer catalysis.88 The polyoxo-tungstate catalyst [π-C5H5NC16H33]3[PO4(WO3)4] (42) forms a soluble, active species in the presence of H2O2. At the end of the reaction, when the oxidant is consumed, the complex precipitates from the reaction mixture and can easily be reused. Moreover, by coupling the H2O2 production process to the 2-ethylanthraquinone–2-ethylanthrahydroquinone redox process, the catalytic epoxidation of propylene occurs without relevant byproduct formation89 and with constant yield of propylene oxide (around 85%) over 3 cycles. Another environmentally friendly route to propylene oxide was found by Mizuno et al.34 The silicotungstate compound (11) exhibits a very good selectivity (>99%) for the epoxide in the oxidation of propylene with H2O2. After a reaction time of 8 h, a yield of 90% propylene oxide is achieved, with a reaction temperature only slightly above room temperature (32 °C). A well-performing catalyst was recently reported by Strukul and co-workers.90 The Pt-based fluorinated complex (43) (Fig. 6) showed excellent catalytic activity with H2O2 as an oxidant (98% yield, 100% epoxide selectivity). However, the need for very low reaction temperatures (−10 °C) as well as the high cost of the catalyst excludes this system from further development for an industrial bulk chemical production. In 2007, Subramaniam et al. presented a novel biphasic reaction for propylene oxide synthesis.91 The oxidation is catalyzed by a MTO–H2O2 system in methanol, and they claim propylene oxide yields exceeding 98%. The reaction proceeds at near-ambient temperature, however, the need for high pressures of gaseous N2 (20 bar), in order to enhance the propylene solubility in the liquid phase, presents a major drawback for industrial application.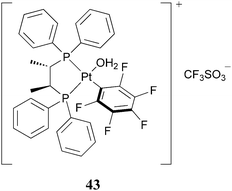 | ||
| Fig. 6 A fluorinated Pt-catalyst for propylene epoxidation. | ||
Conclusion
Catalytic epoxidations of olefins, particularly in a homogeneous phase, are among the best studied reactions in molecular (transition metal) catalysis. A broad scope of different transition metals, among which are Ti-, V-, Cr-, Mo-, W-, Mn-, Re-, Fe- and Ru-catalysts, using quite a wide range of different oxidants, have been presented in the literature so far. They are usually capable of forming stable epoxides in good to excellent yields without diol formation – the most likely side reaction. Meanwhile, a good range of different olefins has been tested, from cyclic olefins (which are quite easy to epoxidize) to acyclic/terminal olefins (usually challenging for epoxidation). Also, numerous asymmetric epoxidations have been presented so far, giving good enantiomeric excesses. However, most reports are constricted to a one-time use of the catalyst, which is, presumably, in most cases destroyed upon workup/product separation. The catalysts, which have been shown to exhibit a good activity when immobilized (ionic liquids give better results than catalysts anchored/grafted to solid supports), yet have to prove themselves to (a) be efficient without activity loss for significantly more than 10, preferably for more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 runs, and (b) be cheap. According to the authors' opinion, these restrictions – catalyst recycling and price – are the drawbacks which homogeneous epoxidation catalysts have to overcome if they want to be competitive beyond academic interest. Thus, a high TOF is attractive for academic reports, however, for industrial purposes, a high TON (i.e. long lifetime) is of more importance. Further, the catalyst is preferably air-stable – a feature that many transition metal complexes lack – and displays a good catalytic performance at temperatures around 25 °C. Ideally, the oxidant used comes from a recyclable source (cumene hydroperoxide), is cheap (O2) or forms benign byproducts (H2O2). Particularly in the epoxidation of ethylene and propylene, remarkably little has been published, in comparison to the high number of reports on the epoxidation of easy-to-oxidize olefins (e.g. cyclooctene). Simple organometallic compounds, such as MTO, cover a wide range of olefin substrates. MTO seems to be one of the most potent catalysts. However, its high price (price of rhenium itself, but also the price of the somewhat demanding synthesis) still does not render it interesting for industrial purposes, even more as it is not suitable for asymmetric catalysis.
000 runs, and (b) be cheap. According to the authors' opinion, these restrictions – catalyst recycling and price – are the drawbacks which homogeneous epoxidation catalysts have to overcome if they want to be competitive beyond academic interest. Thus, a high TOF is attractive for academic reports, however, for industrial purposes, a high TON (i.e. long lifetime) is of more importance. Further, the catalyst is preferably air-stable – a feature that many transition metal complexes lack – and displays a good catalytic performance at temperatures around 25 °C. Ideally, the oxidant used comes from a recyclable source (cumene hydroperoxide), is cheap (O2) or forms benign byproducts (H2O2). Particularly in the epoxidation of ethylene and propylene, remarkably little has been published, in comparison to the high number of reports on the epoxidation of easy-to-oxidize olefins (e.g. cyclooctene). Simple organometallic compounds, such as MTO, cover a wide range of olefin substrates. MTO seems to be one of the most potent catalysts. However, its high price (price of rhenium itself, but also the price of the somewhat demanding synthesis) still does not render it interesting for industrial purposes, even more as it is not suitable for asymmetric catalysis.
Acknowledgements
SAH thanks the TUM Graduate School for financial support.Notes and references
- F. Cavani and J. H. Teles, ChemSusChem, 2009, 2, 508 CrossRef CAS.
- Catalysts for fine chemical synthesis regio- and stereo-controlled oxidations and reductions, ed. S. M. Roberts and J. Whittall, John Wiley Sons, Ltd., England, 2007, vol. 5 Search PubMed.
- T. A. Nijhuis, M. Makkee, J. A. Moulijn and B. M. Weckhuysen, Ind. Eng. Chem. Res., 2006, 45, 3447 CrossRef CAS.
- T. Katsuki and K. B. Sharpless, J. Am. Chem. Soc., 1980, 102, 5974 CrossRef CAS.
- (a) K. Srinivasan, P. Michaud and J. K. Kochi, J. Am. Chem. Soc., 1986, 108, 2309 CrossRef CAS; (b) W. Zhang, J. L. Loebach, S. R. Wilson and E. N. Jacobsen, J. Am. Chem. Soc., 1990, 112, 2801 CrossRef CAS.
- (a) W. A. Herrmann, R. W. Fischer and D. W. Marz, Angew. Chem., Int. Ed., 1991, 30, 1638 CrossRef; (b) C. C. Romão, F. E. Kühn and W. A. Herrmann, Chem. Rev., 1997, 97, 3197 CrossRef; (c) F. E. Kühn, A. Scherbaum and W. A. Herrmann, J. Organomet. Chem., 2004, 689, 4149 CrossRef.
- K. A. Jørgensen, Chem. Rev., 1989, 89, 431 CrossRef.
- B. S. Lane and K. Burgess, Chem. Rev., 2003, 103, 2457 CrossRef CAS.
- P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410 RSC.
- T. Katsuki, Synlett, 2003, 281 CrossRef CAS.
- (a) F. E. Kühn, A. M. Santos and M. Abrantes, Chem. Rev., 2006, 106, 2455 CrossRef; (b) F. E. Kühn, A. M. Santos and W. A. Herrmann, Dalton Trans., 2005, 2483 RSC.
- M. K. Trost and R. G. Bergman, Organometallics, 1991, 10, 1172 CrossRef CAS.
- N. Grover and F. E. Kühn, Curr. Org. Chem., 2012, 16, 16 CrossRef CAS.
- J. H. Espenson, Chem. Commun., 1999, 479 RSC.
- J. J. Haider, R. M. Kratzer, W. A. Herrmann, J. Zhao and F. E. Kühn, J. Organomet. Chem., 2004, 689, 3735 CrossRef CAS.
- J. M. Brunel, Chem. Rev., 2005, 105, 857 CrossRef CAS.
- M. Bougauchi, S. Watanabe, T. Arai, H. Sasai and M. Shibasaki, J. Am. Chem. Soc., 1997, 119, 2329 CrossRef CAS.
- Y. Shi, Acc. Chem. Res., 2004, 37, 488 CrossRef CAS.
- O. A. Wong and Y. Shi, Chem. Rev., 2008, 108, 3958 CrossRef CAS.
- A. Russo, C. De Fusco and A. Lattanzi, ChemCatChem, 2012, 4, 901 CrossRef CAS.
- A. Corma and H. Garcia, Adv. Synth. Catal., 2006, 348, 1391 CrossRef CAS.
- J. Muzart, Adv. Synth. Catal., 2006, 348, 275 CrossRef CAS.
- (a) D. Betz, P. Altmann, M. Cokoja, W. A. Herrmann and F. E. Kühn, Coord. Chem. Rev., 2011, 255, 1518 CrossRef CAS; (b) M. Crucianelli, R. Saladino and F. De Angelis, ChemSusChem, 2010, 3, 524 CrossRef CAS.
- S. Krackl, A. Company, S. Enthaler and M. Drieß, ChemCatChem, 2011, 3, 1186 CrossRef CAS.
- D. Betz, A. Raith, M. Cokoja and F. E. Kühn, ChemSusChem, 2010, 3, 559 CrossRef CAS.
- P. Altmann, M. Cokoja and F. E. Kühn, Eur. J. Inorg. Chem., 2012, 3235 CrossRef CAS.
- M. Bagherzadeh, L. Tahsini, R. Latifi and L. K. Woo, Inorg. Chim. Acta, 2009, 362, 3698 CrossRef CAS.
- A. Günyar, D. Betz, M. Drees, E. Herdtweck and F. E. Kühn, J. Mol. Catal. A: Chem., 2010, 331, 117 CrossRef.
- P. M. Reis, C. A. Gamelas, J. A. Brito, N. Saffon, M. Gómez and B. Royo, Eur. J. Inorg. Chem., 2011, 666 CrossRef CAS.
- M. Abrantes, A. M. Santos, J. Mink, F. E. Kühn and C. C. Romão, Organometallics, 2003, 22, 2112 CrossRef CAS.
- J. A. Schachner, P. Traar, C. Sala, M. Melcher, B. N. Harum, A. F. Sax, M. Volpe, F. Belaj and N. C. Mösch-Zanetti, Inorg. Chem., 2012, 51, 7642 CrossRef CAS.
- A. Rezaeifard, I. Sheikhshoaie, N. Monadi and H. Stoeckli-Evans, Eur. J. Inorg. Chem., 2010, 799 CrossRef CAS.
- I. C. M. S. Santos, F. A. A. Paz, M. M. Q. Simões, M. G. P. M. S. Neves, J. A. Cavaleiro, J. Klinowski and A. M. Cavaleiro, Appl. Catal., A, 2008, 351, 166 CrossRef CAS.
- K. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi and N. Mizuno, Science, 2003, 300, 964 CrossRef CAS.
- V. Madhu, B. Ekambaram, L. J. W. Shimon, Y. Diskin, G. Leitus and R. Neumann, Dalton Trans., 2010, 39, 7266 RSC.
- N. Gharah, S. Chakraborty, A. K. Mukherjee and R. Bhattacharyya, Inorg. Chim. Acta, 2009, 362, 1089 CrossRef CAS.
- Y. Nakagawa, K. Kamata, M. Kotani, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2005, 44, 5136 CrossRef CAS.
- W. A. Herrmann, R. M. Kratzer, H. Ding, W. R. Thiel and H. Glas, J. Organomet. Chem., 1998, 555, 293 CrossRef CAS.
- L. Gomez, I. Garcia-Bosch, A. Company, X. Sala, X. Fontrodona, X. Ribas and M. Costas, Dalton Trans., 2007, 5539 RSC.
- P. Comba, H. Wadepohl and S. Wiesner, Eur. J. Inorg. Chem., 2011, 2610 CrossRef CAS.
- B. Bitterlich, K. Schröder, M. K. Tse and M. Beller, Eur. J. Org. Chem., 2008, 4867 CrossRef CAS.
- P. Gallezot, Catal. Today, 2007, 121, 76 CrossRef CAS.
- C. Sell, The Chemistry of Fragrance: From Perfumer to Consumer, The Royal Society of Chemistry, UK, 2nd edn, 2006 Search PubMed.
- C. Fischer, Food Flavors: Biology and Chemistry, The Royal Society of Chemistry, UK, 1997 Search PubMed.
- P. A. Wender and T. P. Mucciaro, J. Am. Chem. Soc., 1992, 114, 5878 CrossRef CAS.
- D. H. Grayson, Nat. Prod. Rep., 1998, 15, 439 RSC.
- M. Majewski and G. W. Bantle, Synth. Commun., 1992, 22, 23 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS.
- T. R. Boehlow and C. D. Spilling, Tetrahedron Lett., 1996, 37, 2717 CrossRef CAS.
- L. Salles, J.-M. Brégeault and R. Thouvenot, C. R. Acad. Sci., Paris Ser. IIc: Chim., 2000, 3, 183 CrossRef CAS.
- J. Cubillos, M. Vargas, J. Reyes, A. Villa and C. M. de Correa, Chirality, 2010, 22, 403 CAS.
- K. Ravikumar, F. Barbier, J.-P. Bégué and D. Bonnet-Delpon, Tetrahedron, 1998, 54, 7457 CrossRef CAS.
- T. Michel, M. Cokoja, V. Sieber and F. E. Kühn, J. Mol. Catal. A: Chem., 2012, 358, 159 CrossRef CAS.
- T. Michel, D. Betz, M. Cokoja, V. Sieber and F. E. Kühn, J. Mol. Catal. A: Chem., 2011, 340, 9 CrossRef CAS.
- A. L. de Villa, D. E. D. Vos, C. C. de Montes and P. A. Jacobs, Tetrahedron Lett., 1998, 39, 8521 CrossRef.
- H. Rudler, J. R. Gregorio, B. Denise, J.-M. Brégeault and A. Deloffre, J. Mol. Catal. A: Chem., 1998, 133, 255 CrossRef CAS.
- S. Yamazaki, Org. Biomol. Chem., 2010, 8, 2377 CAS.
- T. Michel, M. Cokoja and F. E. Kühn, manuscript in preparation.
- B. Bahramian, V. Mirkhani, S. Tangestaninejad and M. Moghadam, J. Mol. Catal. A: Chem., 2006, 244, 139 CrossRef CAS.
- R. W. Saalfrank, S. Reihs and M. Hug, Tetrahedron Lett., 1993, 34, 6033 CrossRef CAS.
- T. Punniyamurthy, B. Bhatia, M. Reddy, G. C. Maikap and J. Iqbal, Tetrahedron, 1997, 53, 7649 CrossRef CAS.
- K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2024 CrossRef CAS.
- R. M. Hanson and K. B. Sharpless, J. Org. Chem., 1986, 51, 1922 CrossRef CAS.
- A. Lattanzi, P. Iannece and A. Scettri, Tetrahedron Lett., 2002, 43, 5629 CrossRef CAS.
- W. Zhang, A. Basak, Y. Kosugi, Y. Hoshino and H. Yamamoto, Angew. Chem., Int. Ed., 2005, 44, 4389 CrossRef CAS.
- J. Benet-Buchholz, P. Comba, A. Llobet, S. Roeser, P. Vadivelu, H. Wadepohl and S. Wiesner, Dalton Trans., 2009, 5910 RSC.
- S. Koya, Y. Nishioka, H. Mizoguchi, T. Uchida and T. Katsuki, Angew. Chem., Int. Ed., 2012, 124, 8368 CrossRef.
- H. Tanaka, H. Nishikawa, T. Uchida and T. Katsuki, J. Am. Chem. Soc., 2010, 132, 12034 CrossRef CAS.
- W. Mägerlein, C. Dreisbach, H. Hugl, M. K. Tse, M. Klawonn, S. Bhor and M. Beller, Catal. Today, 2007, 121, 140 CrossRef.
- H. Nishiyama and Y. Motoyama, Chem. Commun., 1997, 1863 RSC.
- F. Gelalcha, G. Anilkumar, M. Tse, A. Brückner and M. Beller, Chem.–Eur. J., 2008, 14, 7687 CrossRef CAS.
- K. Matsumoto, Y. Sawada, B. Saito, K. Sakai and T. Katsuki, Angew. Chem., Int. Ed., 2005, 44, 4935 CrossRef CAS.
- Y. Shimada, S. Kondo, Y. Ohara, K. Matsumoto and T. Katsuki, Synlett, 2007, 2445 CAS.
- A. Berkessel, M. Brandenburg, E. Leitterstorf, J. Frey, J. Lex and M. Schäfer, Adv. Synth. Catal., 2007, 349, 2385 CrossRef CAS.
- A. U. Barlan, A. Basak and H. Yamamoto, Angew. Chem., Int. Ed., 2006, 45, 5849 CrossRef CAS.
- P. Pietikäinen, J. Mol. Catal. A: Chem., 2001, 165, 73 CrossRef.
- S. Watanabe, Y. Kobayashi, T. Arai, H. Sasai, M. Bougauchi and M. Shibasaki, Tetrahedron Lett., 1998, 39, 7353 CrossRef CAS.
- K. Daikai, M. Kamaura and J. Inanaga, Tetrahedron Lett., 1998, 39, 7321 CrossRef CAS.
- T. Nemoto, T. Ohshima, K. Yamaguchi and M. Shibasaki, J. Am. Chem. Soc., 2001, 123, 2725 CrossRef CAS.
- K. Daikai, T. Hayano, R. Kino, H. Furuno, T. Kagawa and J. Inanaga, Chirality, 2003, 15, 83 CrossRef CAS.
- A. Minatti and K. H. Dötz, Eur. J. Org. Chem., 2006, 268 CrossRef CAS.
- P. A. Kilty and W. M. H. Sachtler, Catal. Rev., 1974, 10, 1 CAS.
- H.-J. Lee, M. Ghanta, D. H. Busch and B. Subramaniam, Chem. Eng. Sci., 2010, 65, 128 CrossRef CAS.
- J. Kollar, Catalytic epoxidation of an olefinically unsaturated compound using an organic hydroperoxide as an epoxidizing agent, Halcon International, Inc., U.S. Patent 3,350,422, 1967 Search PubMed.
- J. Kollar, Epoxidation process, Halcon International, Inc., U.S. Patent 3,351,635, 1967 Search PubMed.
- M. N. Sheng and J. G. Zajacek, Method of Producing Epoxides, The Atlantic Richfield Company, G.B. Patent 1,136,923, 1968 Search PubMed.
- I. Yamanaka, K. Nakagaki and K. J. Otsuka, Chem. Commun., 1995, 1185 RSC.
- X. Zuwei, Z. Ning, S. Yu and L. Kunlan, Science, 2001, 292, 1139 CrossRef CAS.
- Z. Ning, X. Zuwei, C. Guoying and G. Shuang, Appl. Catal., A, 2003, 250, 239 CrossRef.
- M. Colladon, A. Scarso, P. Sgarbossa, R. A. Michelin and G. Strukul, J. Am. Chem. Soc., 2006, 128, 14006 CrossRef CAS.
- H.-J. Lee, T.-P. Shi, D. H. Busch and D. Subramaniam, Chem. Eng. Sci., 2007, 62, 7282 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |