Preparation of Au/TiO2 exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation from organic and inorganic compounds under irradiation of visible light†
Atsuhiro
Tanaka
,
Satoshi
Sakaguchi
,
Keiji
Hashimoto
and
Hiroshi
Kominami
*
Department of Applied Chemistry, Faculty of Science and Engineering, Kinki University, Kowakae, Higashiosaka Osaka 577-8502, Japan. E-mail: hiro@apch.kindai.ac.jp
First published on 15th March 2012
Abstract
Au/TiO2 samples exhibiting stronger photoabsorption at around 550 nm due to surface plasmon resonance were prepared by using a multi-step photodeposition method and the samples exhibited higher levels of activity for H2 production from various compounds such as 2-propanol, ethanol and ammonia in aqueous suspensions under visible light irradiation.
The development of a clean and renewable energy carrier has become a subject of high priority. Hydrogen (H2) production by semiconductor photocatalysts under irradiation of visible light has attracted much attention because it offers a promising way for clean, low-cost and environmentally friendly production of H2 by solar light.1 Recently, it has been reported that electron transfer from gold (Au) nanoparticles to titanium(IV) oxide (TiO2) occurred under irradiation of visible light (λ = ca. 550 nm) due to surface plasmon resonance (SPR).2a,b Some research groups have since found that chemical reactions, i.e., oxidation of organic compounds, occurred in an aqueous suspension of Au supported on TiO2 (Au/TiO2), a new type of catalytic material responding to visible light different from semiconductor photocatalysts, and it was concluded that these reactions were induced by SPR of supported Au nanoparticles.2c–e We have found that Au nanoparticles supported on cerium(IV) oxide (Au/CeO2) exhibited absorption due to SPR more strongly than did various Au/TiO2 samples with the same Au content and that Au/CeO2 exhibited a much higher level of activity than the activity levels of Au/TiO2 samples in mineralization3a,b and partial oxidation3c of organic compounds. We also succeeded in shift of SPR to a longer length in copper (Cu)–Au/CeO2 and mineralization of organic acids in an aqueous suspension of Cu–Au/CeO2 even under irradiation of visible light of λ = 700 nm.4 Very recently, Silva et al.5 reported production of H2 from EDTA or methanol in aqueous suspensions of Au/TiO2 (in which Degussa P 25 TiO2 was used) under irradiation of visible light and proposed that H2 was formed by reduction of protons with electrons in the conduction bands of TiO2 injected from Au nanoparticles. Since, unfortunately, their Au/TiO2 samples were slightly deactivated within several hours of photoirradiation, understanding photocatalytic properties of Au/TiO2 and preparation of Au/TiO2 samples with high stability and high levels of activity are highly important. As is apparent from several studies, supported Au nanoparticles (Au/TiO2 and Au/CeO2) have various possibilities as photocatalytic materials utilizing visible light.
Still TiO2 attracted our interest for use as a support for Au nanoparticles because TiO2 is inexpensive and its physical properties can be easily controlled and we think that a combination of Au nanoparticles exhibiting strong SPR and a TiO2 sample suitable for Au fixation, electron transfer and substrate adsorption is important for higher levels of activity in photoinduced reactions. However, effects of the method for Au deposition and size distribution of Au particles on the activity in photoinduced reactions have not been reported, although there are many papers reporting Au photodeposition on TiO2 and the effects on (thermo)catalytic activities of Au/TiO2. In this study, by using a multi-step (MS) photodeposition method4 in which addition of metal sources and photodeposition of the metals on semiconductor particles were repeated several times to obtain a desired metal loading, we succeeded in preparation of Au nanoparticles supported on TiO26 (MS-Au/TiO2) exhibiting SPR absorption stronger than that of Au/TiO2 prepared by using a single-step photodeposition method (SS-Au/TiO2). Here we report a larger rate of photoinduced H2 production from various substrates (2-propanol, ethanol, methanol, ammonia and benzyl alcohol) in aqueous suspensions of MS-Au/TiO2 samples under irradiation of visible light.
Fig. 1(a) shows a TEM photograph of SS-(1.00)Au/TiO2. The amount of Au loaded was generally 1.00 wt% and is shown in parentheses before Au if necessary. Small Au particles were observed in the TEM photograph of SS-(1.00)Au/TiO2 and the average diameter of Au particles was determined to be 1.2 nm, suggesting that the SS photodeposition method can be used to highly disperse Au nanoparticles on the surface of TiO2. Fig. 1(b)–(e) show TEM photographs of MS-Au/TiO2 samples on which 0.25, 0.50, 0.75 and 1.00 wt% of Au was loaded by repeating 0.25 wt% loading of Au. Two types of Au particles having different sizes were observed in the 0.50, 0.75 and 1.00 wt% samples, although the number of larger particles was less than that of smaller particles. As shown in Fig. 1 and 2, the average sizes of larger Au particles gradually increased with increase in times of Au loading. These results indicate that the Au sources added after the first photodeposition in the MS photodeposition method were deposited on Au particles previously formed on the TiO2 surface, resulting in growth of the Au particles. The original size of some of the Au particles formed at the first deposition was preserved and the average particle sizes of MS-(1.00)Au/TiO2 samples were determined to be 1.4 nm and 13 nm, respectively (ESI†, Fig. S1).
 | ||
| Fig. 1 TEM image of (a) SS-(1.00)Au/TiO2, (b) MS-(0.25)Au/TiO2, (c) MS-(0.50)Au/TiO2, (d) MS-(0.75)Au/TiO2, (e) MS-(1.00)Au/TiO2, and (f) sample obtained by post-calcination of MS-(1.00)Au/TiO2 at 500 °C. | ||
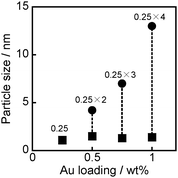 | ||
| Fig. 2 The average size of larger and smaller Au nanoparticles loaded on MS-Au/TiO2 having different Au loadings. Values in the figure are the amounts and the times of Au loading. | ||
Fig. 3(a) shows intensity of photoabsorption at 550 nm of MS-Au/TiO2 having different Au loadings and Fig. 3(b) shows absorption spectra of SS- and MS-(1.00)Au/TiO2 samples. Photoabsorption at 550 nm was attributed to SPR of the supported Au particles as reported previously.2–5 It should be noted that MS-(1.00)Au/TiO2 exhibited much stronger photoabsorption than that of SS-(1.00)Au/TiO2, although the amounts of Au loaded on TiO2 were the same. This result suggests that the intensity of photoabsorption due to SPR of Au was affected by the size of Au nanoparticles. Kowalska et al. reported that particle size and photoabsorption peak of Au supported on TiO2 were affected by properties of TiO2.2d,e
 | ||
| Fig. 3 (a) Intensity of photoabsorption of MS-Au/TiO2 having different Au loadings (circles) and SS-(1.00)Au/TiO2 (square), (b) absorption spectra of SS-(1.00)Au/TiO2 (broken line) and MS-(1.00)Au/TiO2 (solid line), and light intensity of visible light irradiated to reaction systems from a Xe lamp with a Y-48 filter (right axis). Values in the figure are the amounts and the times of Au loading. | ||
Fig. 4(a) shows time courses of evolution of H2 from 2-propanol in aqueous suspensions of SS- and MS-(1.00)Au/TiO2 under irradiation of visible light in the absence of oxygen. For comparison, results for Au-free TiO2 are also shown in the figure. Just after irradiation of visible light, H2 was evolved from suspensions of SS- and MS-Au/TiO2, while no H2 was formed from the suspension of Au-free TiO2. Since H2 increased linearly with photoirradiation time, the rates of H2 evolution were determined to be 0.15 and 2.7 μmol h−1, respectively, i.e., MS-Au/TiO2 exhibited a rate of H2 formation ca. 20-times larger than that of SS-Au/TiO2. MS-(0.50)Au/TiO2 and MS-(0.75)Au/TiO2 with smaller Au contents exhibited rates (0.44 and 0.98 μmol h−1, respectively) larger than that of SS-(1.00)Au/TiO2. Intense SPR absorption of MS-Au/TiO2 due to the larger Au particles is one of the main reasons of the extraordinarily large activity for H2 production. However, the difference in activities of two Au/TiO2 samples was much larger than the difference in SPR absorption (Fig. 3). It has been reported that Au particles having a diameter of around 2 nm loaded on TiO2 worked as reduction centers for H2 evolution in addition to SPR photoabsorption.5 Post-calcination has been widely applied to control the particle size of Au loaded on various supports. When MS-Au/TiO2 was calcined at 500 °C in air, smaller Au particles having an average diameter of 1.4 nm disappeared (Fig. 1f), suggesting that these particles were unified with larger Au particles. As shown in Fig. 4(b), the rate of H2 formation decreased with elevating calcination temperature. These results suggest that smaller Au particles of MS-Au/TiO2 uncalcined might have important roles such as charge separation and H2 evolution other than photoabsorption, although we do not have any other evidence. The yields of H2 reached 3.0 and 54.2 μmol after 20 h in the case of SS- and MS-Au/TiO2, respectively, while the yields of acetone in the liquid phase were 3.98 and 54.9 μmol, respectively. Good agreement with yields of H2 and acetone in both samples shows that stoichiometric dehydrogenation of 2-propanol occurred as expressed in eqn (1).
| (CH3)2CHOH → (CH3)2CO + H2 | (1) |
No gas was evolved in the dark between 20 and 26 h, indicating that no thermocatalytic H2 formation occurred in both cases of SS- and MS-Au/TiO2 under the present conditions. To evaluate the stability of Au/TiO2 in H2 production from 2-propanol, Au/TiO2 was used again. Irradiation of visible light to the reaction mixtures again induced evolution of H2 in both cases and the formations continued from 26 h to 46 h without deactivation. The total amount of H2 (ca. 105 μmol) was ca. 42-times larger than the amount of Au (2.5 μmol, corresponding to 1.0 wt%) loaded on TiO2, indicating that the H2 production observed under the present irradiation conditions was not a quantitative reagent reaction between Au and 2-propanol but a (photo)catalytic reaction.
 | ||
| Fig. 4 (a) Time courses of evolution of H2 from 2-propanol in aqueous suspensions of TiO2 (triangles), SS-(1.00)Au/TiO2 (open circles) and MS-(1.00)Au/TiO2 (closed circles) under irradiation of visible light from a Xe lamp with a Y-48 filter and (b) effect of post-calcination temperature on the rate of H2 evolution. | ||
To extend the possibility of Au/TiO2 for H2 formation under visible light, various compounds soluble in water were used as substrates. Ethanol, glycerin and ammonia were chosen as a typical biomass, a by-product in biomass utilization (saponification and transesterification of fats and oils) and biomass waste that is mainly included in excretion, respectively. Table 1 summarizes rates of H2 production from these compounds in aqueous suspensions of SS- and MS-Au/TiO2 under visible light irradiation. Evolution of H2 was observed in all compounds, indicating that biomass-related compounds are strong candidates as an H2 source when Au/TiO2 is used under visible light irradiation. Larger rates of H2 formation were obtained in all of the Au/TiO2 samples prepared using the MS photodeposition method for Au loading. In the reaction of ammonia, 10 μmol of H2 was evolved (entry 5) along with 3.2 μmol of N2. Since the amount of N2 was in good agreement with that of H2, ammonia was decomposed stoichiometrically to H2 and N2 as shown in eqn (2).
| 2NH3 → N2 + 3H2 | (2) |
In the reaction of benzyl alcohol in an aqueous suspension of MS-Au/TiO2, 4.6 μmol of H2 was formed over a period of 25 h (entry 6). Since almost the same amount of benzaldehyde (4.2 μmol) was formed in the liquid phase, it can be concluded that dehydrogenation of benzyl alcohol to benzaldehyde occurred as shown in eqn (3) under the present conditions.
| C6H5CH2OH → C6H5CHO + H2 | (3) |
Since benzaldehyde is used widely in various chemical industries, this reaction has two meanings, i.e., evolution of H2 and selective production of benzaldehyde under irradiation of visible light.
| Entry | Substratea | Time/h | H2 production/μmol | |
|---|---|---|---|---|
| SS | MS | |||
| a 50 vol% alcohol solutions and 14 wt% ammonia solution. b External quantum efficiency was roughly estimated to be 0.05% based on incident photons from 470 to 720 nm. | ||||
| 1 | 2-Propanol | 5 | 0.73 | 13b |
| 2 | Methanol | 5 | 0.84 | 17 |
| 3 | Ethanol | 5 | 0.59 | 12 |
| 4 | Glycerin | 10 | 0.63 | 14 |
| 5 | Ammonia | 10 | 0.73 | 10 |
| 6 | Benzyl alcohol | 25 | 0.34 | 4.6 |
In conclusion, we succeeded in the formation of H2 from various compounds including biomass-related compounds (2-propanol, methanol, ethanol, ammonia and benzyl alcohol) in aqueous suspensions of Au/TiO2 under visible light irradiation. By using the MS photodeposition method, Au/TiO2 samples exhibiting stronger photoabsorption at around 550 nm due to SPR and having higher levels of activity for H2 production were prepared.
Notes and references
-
(a) I. Tsuji, H. Kato and A. Kudo, Angew. Chem., Int. Ed., 2005, 44, 3565 CrossRef CAS
; (b) K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef CAS
; (c) K. Maeda, A. Xiong, T. Yoshinaga, T. Ikeda, N. Sakamoto, T. Hisatomi, M. Takashima, D. Lu, M. Kanehara, T. Setoyama, T. Teranishi and K. Domen, Angew. Chem., Int. Ed., 2010, 49, 4096 CrossRef CAS
; (d) X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS
.
-
(a) Y. Tian and T. Tatsuma, J. Am. Chem. Soc., 2005, 127, 7632 CrossRef CAS
; (b) A. Furube, L. Du, K. Hara, R. Katoh and M. Tachiya, J. Am. Chem. Soc., 2007, 129, 14852 CrossRef CAS
; (c) S. Naya, M. Teranishi, T. Isobe and H. Tada, Chem. Commun., 2010, 46, 815 RSC
; (d) E. Kowalska, R. Abe and B. Ohtani, Chem. Commun., 2009, 241 RSC
; (e) E. Kowalska, O. O. P. Mahaney, R. Abe and B. Ohtani, Phys. Chem. Chem. Phys., 2010, 12, 2344 RSC
.
-
(a) H. Kominami, A. Tanaka and K. Hashimoto, Chem. Commun., 2010, 46, 1287 RSC
; (b) H. Kominami, A. Tanaka and K. Hashimoto, Appl. Catal., A, 2011, 397, 121 CrossRef CAS
; (c) A. Tanaka, K. Hashimoto and H. Kominami, Chem. Commun., 2011, 47, 10446 RSC
.
- A. Tanaka, K. Hashimoto and H. Kominami, ChemCatChem, 2011, 3, 1619 CrossRef CAS
.
- C. G. Silva, R. Juarez, T. Marino, R. Molinari and H. Garcia, J. Am. Chem. Soc., 2011, 133, 595 CrossRef
.
-
(a) H. Kominami, M. Kohno, Y. Takeda, M. Inoue, T. Inui and Y. Kera, Ind. Eng. Chem. Res., 1999, 38, 3925 CrossRef CAS
; (b) H. Kominami, S.-y. Murakami, J.-i. Kato, Y. Kera and B. Ohtani, J. Phys. Chem. B, 2002, 106, 10501 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and Fig. S1. See DOI: 10.1039/c2cy20108a |
| This journal is © The Royal Society of Chemistry 2012 |
