Nanotwins in polycrystalline Cu7S4 cages: highly active architectures for enhancing photocatalytic activities†
Shaodong
Sun
,
Xiaoping
Song
,
Dongchu
Deng
,
Xiaozhe
Zhang
and
Zhimao
Yang
*
School of Science, State Key Laboratory for Mechanical Behavior of Materials, MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, Xi'an Jiaotong University, Xi'an 710049, Shaanxi, People's Republic of China. E-mail: zmyang@mail.xjtu.edu.cn
First published on 15th May 2012
Abstract
We present the first evidence for the synthesis of nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells. The photocatalytic superiority of these cages is attributed to the presence of a larger number of nanotwinned building blocks, which exhibited higher adsorption ability and catalytic activity for enhancing photodegradation of methylene blue (MB) dye.
Introduction
Hollow three-dimensional (3D) nanostructures, a typical type of promising structures with large surface area, good conductivity and permeability for charge and mass (gas) transport, have been attracting increasing interest due to their potential applications in catalysts, lithium ion batteries, sensors, drug-delivery carriers and so on.1–9 To accurately tailor the features of hollow structures has been an exciting challenge because a thorough understanding of the formation mechanism and growth process has significant scientific value. So far, hollow architectures with different subunits can be successfully synthesized by precisely controlling the nucleation and growth process by a “bottom-up” self-assembly technique. The size, morphology and crystallinity of hollow cages can significantly affect their chemicophysical properties. Many recent efforts have been to develop the size- and shape-tailored fabrication of various hollow architectures because of their special size- and shape-dependent effects. However, it still remains a scientific curiosity and a great challenge to employ facile methods to synthesize well-defined hollow superstructures with controllable crystallinity.As a non-stoichiometric p-type semiconductor10 (band gap 1.2–2.0 eV) with unique optical, electric and thermal properties, copper sulfide is a prospective material with applications in solar cells, optical filters, nanoswitches, thermoelectric and photoelectric transformers, superconductors, gas sensors and lithium-ion batteries.11 So far, many synthesis strategies have been focused on the shape-controlled synthesis of various copper sulfide architectures, such as nanorods,12 nanotubes,13 nanoflakes,14 nanotrees,15 nanowalls,16 hollow spheres17 and nanocages.18 Although various copper sulfide hollow cages with single-crystalline shells (such as cubes, octahedra, and multi-facet polyhedra) have been successfully synthesized by using single-crystalline cuprous oxide (Cu2O) crystals as sacrificial templates,1–3 to the best of our knowledge, there are no reports on the synthesis of nanotwins in copper sulfide hollow polyhedral architectures exposed to polycrystalline shells. It is proposed that the structure-dependent tailoring which determines the surface atomic arrangement and surface energy is of great importance for the prospective application of copper sulfides.
In a typical Cu2O crystal lattice, the surface atom states of {110}, {111} and {100} facets are wholly different.11 The {110} and {111} facets of Cu2O crystals are formed by both “Cu” and “O” atoms, and surface “Cu” atoms with dangling bonds on {110} and {111} facets can make them positively charged, so {110} and {111} facets can be facilely protected by a negatively charged agent in the reaction environment.11 Furthermore, it is seen that the distance between two “Cu” atoms of {110} facets is about half of that in {111} facets, implying that the number and density of “Cu” dangling bonds in the {110} facets are higher than those of the {111} facets, so the {110} facets possess more dangling bonds and higher surface energy than {111} facets. However, the {100} facets are predominated by “Cu” or “O” atoms only, leading to the electrically neutral state of {100} facets usually.11 Thus the protection of {100} facets is weak, and it is facile to be modified. It has been demonstrated that the surface reaction process between “S” and “O” atoms can be modified by adjusting the solvent molecules, which can change the features of different crystallographic facets in solvents of different polarity, leading to the formation of shells with different structures.11,19 Finally, the solvent-dependent synthesis of polyhedral 26-facet CuxSy cages with different stoichiometries and microstructures can be artificially achieved.
In the recent report,11,19 we have reported the synthesis of unique polyhedral 26-facet CuS microcages decorated with nanotwinned, mesostructural and single crystalline shells using a facile, template-free, sacrificial, polyhedral 26-facet Cu2O templates solution route.11 However, it has been demonstrated that single crystalline-like polyhedral 26-facet Cu7S4 cages exposed to amazingly unique nanotwinned structures as building blocks were artificially achieved by a facile, ethanol-assisted, sacrificial Cu2O templates approach, which can exhibit higher photocatalytic activity for enhancing degradation of methylene blue (MB).19 Especially, Yao and co-workers have recently reported that the employment of a great number of nanotwin structures in semiconductors (Cd1−xZnxS) could significantly improve their photocatalytic activities, because the twins inside a photocatalyst can not only keep the transport property of free charges as in perfect crystals (due to their highly ordered structures), but also prevent the re-combination of holes and electrons (because of the possibility of forming an electrostatic field).19 Based on the above excellent literature example, it is proposed that the introduction of a larger number of nanotwinned building blocks into copper sulfide cages would bring about excellent photochemical activities. Consequently, the crystallinity-controlled synthesis of nanotwinned polyhedral hollow copper sulfide cages wholly exposed to polycrystalline shells is an interesting and challenging topic for both fundamental study and potential applications. To date, the relevant experimental investigation is still unavailable.
Herein, we demonstrate the first evidence of the synthesis of nanotwinned polyhedral 26-facet Cu7S4 cages with polycrystalline shells. The formation of polycrystalline nanotwinned polyhedral 26-facet Cu7S4 cages not only provides a facile protocol to synthesize novel copper sulfide structures with enhanced photochemical activity, further enriching the family of copper sulfide nanostructures, but also gives a good opportunity to understand the significance for the investigation of the potential application of as-prepared nanotwinned 26-facet Cu7S4 cages with polycrystalline shells in the field of photocatalytic hazardous pollutants.
Results and discussion
Our strategy to achieve the fabrication of nanotwinned polyhedral 26-facet Cu7S4 cages with polycrystalline shells is based on a Cu2O-templated growth/etching route, which is believed to be the result of the Kirkendall effect.1–3 They can be synthesized by a three-step process, i.e. (i) the formation of single-crystalline polyhedral Cu2O templates; (ii) the synthesis of Cu2O/Cu7S4 core/shell structures in a mixed glycol (EG) solution composed of sodium sulfide and sodium hydroxide, namely, after addition of Cu2O templates into the reaction system, a thin copper sulfide layer composed of many nanoplates would be formed immediately on the surface of Cu2O templates, as evidenced by a fast color change of the Cu2O templates from dark red to black. Further reaction depends on the diffusion of copper or sulfur ions through this copper sulfide interface, thus leading to the formation of voids in the particles; (iii) the inner Cu2O cores are dissolved completely with an ammonia solution. In a typical synthesis, 26-facet Cu2O particles (0.6 g) were added to a mixed EG solution (150 mL) composed of Na2S (0.36 g) and NaOH (0.006 g) at room temperature and ambient pressure for 10 min under magnetic stirring. The precipitates were separated by centrifugation, washed with deionized water and ethanol. Afterwards, these Cu2O/Cu7S4 core/shell particles were immersed in ammonia (NH3·H2O) solution (25%) for 72 h to remove the inner Cu2O cores. The dark particles were centrifuged twice in deionized water and anhydrous ethanol, respectively. And finally were dried at 60 °C for 12 hours in a vacuum oven.The powder X-ray diffraction (XRD) pattern was recorded on a Bruker-AXS D8 ADVANCE diffractometer operated at 40 kV voltage and 40 mA current using Cu Kα radiation (λ = 1.5406 Å). The morphology of the powders was investigated by a JEOL (JSM-7000F) field-emission scanning electron microscope (FE-SEM) at an accelerating voltage of 20 kV. The transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) analysis as well as selected-area electron diffraction (SAED) analysis were performed on a JEOL JEM-2100 transmission electron microscope operating at an accelerating voltage of 200 kV. The UV-vis absorption spectra of as-prepared nanotwinned Cu7S4 cages were obtained using a UV/vis/NIR spectrophotometer (Hitachi U-4100).
The catalytic activity experiments of the different kinds of copper sulfide powders for the oxidation and decoloration of the methylene blue (MB) dye with the assistance of hydrogen peroxide (H2O2) were carried out at ambient temperature. The original solution was prepared by adding 0.25 mL H2O2 (30%, w/w) and 0.4 mL MB solution (400 mg L−1) to 100 mL deionized water, then 3 mg copper sulfide powder was added into the solution to form an aqueous dispersion, and was magnetically stirred in the dark for 30 min to ensure establishing an adsorption–desorption equilibrium. Afterwards, the dispersion was irradiated using a 500 W xenon lamp equipped with a filter cutoff (λ ≥ 420 nm) under magnetic stirring using a BL-GHX photochemical reactor. At given time intervals, the dispersions were sampled and centrifuged to separate the catalysts. They were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min (XIANYI TG16-WS centrifuge). UV-vis absorption spectra were recorded at different intervals to monitor the reaction using a UV/vis/NIR spectrophotometer (Hitachi U-4100).
000 rpm for 2 min (XIANYI TG16-WS centrifuge). UV-vis absorption spectra were recorded at different intervals to monitor the reaction using a UV/vis/NIR spectrophotometer (Hitachi U-4100).
Structural analysis of the as-prepared polyhedral cages was carried out using XRD, and the result is shown in Fig. 1. All the diffraction peaks are indexed according to the standard monoclinic structure of Cu7S4 (JPCDS file No. 23-0958) with the C2/m space group. No peaks of impurities such as copper oxides or other copper sulfides were detected, suggesting the high purity of the as-obtained products.
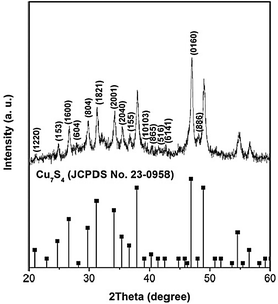 | ||
| Fig. 1 XRD pattern of the as-prepared polyhedral cages. | ||
The morphology and structural characterization of the as-prepared polyhedral Cu7S4 cages was also performed using TEM, HRTEM and SAED investigations. The detailed characterizations of the as-made products are shown in Fig. 2. Fig. 2a shows the low-magnification FESEM image of the as-prepared products, indicating that the particles are uniform and monodisperse 26-facet polyhedral architectures. And it can be obviously seen that the formation of rough surfaces of the shells with well-assembled nanoplate building blocks can be achieved. The partially broken particles (as shown with the white arrow) can also be observed in Fig. 2a, demonstrating that 26-facet cages with hollow interiors are formed. Fig. 2b shows a typical low-magnification TEM image, and it can be observed that the particle is made of three similar kinds of rough shells and void inside, especially an extremely strong contrast difference between their edges (dark) and interiors (bright), suggesting the formation of a cage with a hollow nature. However, this is different from the particles obtained with anhydrous ethanol instead of EG solution which are made of three different kinds of rough shells and void inside, especially an extremely strong contrast difference between their triangles (dark) and quadrangles (bright).20 The corresponding SAED pattern of the area marked with a yellow circle is displayed in the inset of Fig. 2b, indicating that the as-prepared 26-facet Cu7S4 cages possess polycrystalline shells. Fig. 2c shows the high-magnification TEM image of a typical shell of a hollow cage, which implies that the polyhedral 26-facet Cu7S4 hollow cages composed of abundant nanoplate building blocks with irregular alignment are successfully synthesized in the present work. Some of these nanoplates have joined together and disorderly attached to form obvious cavities or mesopores inside the cage shell. The detailed microstructure of a nanoplate building block was further investigated using HRTEM, as shown in Fig. 2d. A high density of planar defects is clearly found, which form a domain structure with nanotwinned planes. The corresponding fast Fourier transform (FFT) of the HRTEM images of the planar defects (Fig. 2d) is shown in the corresponding inset. It is expressed as heavy streaking of the reflections, which is similar to that occurring in the previous report,21 indicating the formation of nanotwinned structures in the nanoplate building blocks. The above observations obviously support that the highly symmetric nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells can be successfully prepared in our synthesis. The possible formation of nanotwinned copper sulfide hollow cages has been discussed in our previous report,20 which may be attributed to the in situ transformation of Cu2O templates with intrinsic difference of surface states in solvents of different polarity. Importantly, it can be found that the amount of the nanotwins of these polycrystalline shells is obviously higher than that of the single crystalline-like shells as shown in a previous report.19
 | ||
| Fig. 2 (a) FESEM image of the as-prepared products; (b) low-magnification TEM image of the as-prepared products, and the inset is the corresponding SAED pattern taken from the area marked with a yellow circle; (c) high-magnification TEM image of a typical polycrystalline shell; (d) HRTEM image of a typical nanoplate building block, and the inset is the corresponding FFT image. | ||
A schematic illustration of the reaction pathways of the solvent-controlled synthesis of copper sulfide cages with double crystallinity is listed in Fig. 3. It can be seen that the crystallinity of the shells of Cu7S4 microcages can be well controlled by adjusting the species of organic additives. When the solvent anhydrous ethanol (EtOH) was used under otherwise the same conditions, the 26-facet Cu7S4 microcages with three different types of single crystalline-like shells can be successfully synthesized, and these results were reported in detail in our recent work.19 However, in the present work when the solvent EG was used instead of EtOH, the nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells were synthesized. A possible mechanism can be discussed as follows. During the formation process of the copper sulfide shells, the diffusion rate of copper ions from the inner to the surface was faster than that of the sulfur ions from the surface to the inner, finally resulting in the formation of voids in the particles due to the Kirkendall effect.17,19 Moreover, the strain energy is likely to be stored in the copper sulfide interfaces during the mass exchange process because of the difference in atomic radius between the sulfur and the oxygen atom. This stored strain energy can be released by forming nanotwins as the growth of nanoplates is completed.22 These high-activity nanotwinned building blocks can be saved in the presence of a capping agent, and they can also drive the spontaneous disordering assembly to form these hierarchical cages because the polarity of EG is less than that of EtOH, which means that the electrical force between any two charges in the EG system is much larger, which is similar to that occurring in TiO2 architectures.23,24 Therefore, it is further demonstrated that the formation of different crystallinities of Cu7S4 cages might be attributed to the aggregation behaviour of nanoplate building blocks during the replacement/etching process in solvents of different polarity.
 | ||
| Fig. 3 A schematic illustration of the reaction pathways of the solvent-controlled synthesis of Cu7S4 cages with different crystallinities. | ||
Very recently, Yao and co-workers have reported that photocatalytic activity is strongly dependent on the separation efficiency of photogenerated electrons and holes in the photocatalysts, especially the amount of nanotwins in the semiconductors can strongly affect the enhancement of their photochemical activities.20 In order to further demonstrate the potential application of the as-synthesized nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells in the photodegradation of organic pollutants, we have investigated their photocatalytic activities by choosing the photocatalytic degradation of MB dye in the presence of hydrogen peroxide. The catalytic reaction was conducted under visible light at room temperature. UV-Vis spectra were used to investigate the adsorption and photocatalytic degradation of MB dye. The characteristic absorption peak at 664 nm of MB was used to monitor the photocatalytic degradation process. It can be obviously seen that the polycrystalline 26-facet Cu7S4 cages have a much better adsorption capacity (Fig. 4). The results show that polycrystalline Cu7S4 cages adsorb about 62.3% of MB in the solution, while single-crystalline-like 26-facet Cu7S4 cages adsorb about 32.8% in 30 min (Fig. 4). Fig. S1a shows the optical absorption spectra of MB tested at different durations without any catalysts (see ESI†), only about 21% of the MB (Fig. 4, Curve A) was degraded after 90 min. In addition, the assistance of H2O2 can be in favor of the bleaching of MB, which is similar to the experiments described in previous reports.25 Fig. S1b shows the optical absorption spectra of MB tested at different durations with commercial copper sulfide powders (see ESI†), and about 52% of the MB (Fig. 4, Curve B) was degraded after 90 min. A better photocatalytic activity of polycrystalline 26-facet Cu7S4 cages than that of the single-crystalline-like 26-facet Cu7S4 cages can be directly also determined from the curves shown in Fig. S1c and Fig. S1d (see ESI†). Fig. S1d (ESI†) shows the optical absorption spectra of MB tested at different intervals in the presence of as-prepared nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells synthesized in the EG system (sample A, as shown in Fig. 2). The intensity of the absorption peak at 664 nm of MB decreased rapidly with the extension of exposure time, suggesting the fast photocatalytic degradation of MB, and about 86% of the MB (Fig. 4, Curve D) was degraded after 90 min. Moreover, we also carried out comparison tests using the previously reported nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within single crystalline-like shells synthesized in an anhydrous ethanol (EtOH) system (sample B). We found that the decrease in intensity of the absorption peak at 664 nm of MB was slower than that for the as-prepared Cu7S4 microcages wholly exposed to nanotwinned structures (Fig. S1c, ESI†), and about 71% of the MB (Fig. 4, Curve C) was degraded after 90 min, which demonstrates that the amount of nanotwinned structures plays a key role in accelerating the photodegradation of MB dye. The decomposition of the MB aqueous solution at 90 min in the presence of above two samples is as follows: nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells (86%) > nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within single crystalline-like shells (71%) > commercial powders (52%).
 | ||
| Fig. 4 A plot of the extent of photodegradation of MB by different catalysts under natural light. Curve A: without catalysts; Curve B: commercial powders; Curve C: nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within single crystalline-like shells; Curve D: the as-prepared nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells. | ||
The possible photodegradation mechanism has been discussed in our previous report,19 which involves the electron–hole pair separation and subsequent scavenging of the electrons and trapping of holes by H2O2 molecules, leading to the formation of the active oxidants. And these photogenerated oxidant species are in favor of oxidizing organic contaminants due to their high oxidative capacities.25 When the Cu7S4 cages were irradiated with UV light in the presence of H2O2, the acceleration in photodecomposition of H2O2 over Cu7S4 cages would be generated. Holes in the conduction band (CB) would be excited to the valence band (VB), with simultaneous formation of electrons in the CB.26 The electrons and holes can be captured by H2O2 molecules, resulting in the formation of the oxidants (eqn (1)–(4)).19
| Cu7S4 + hν → hvb+ + ecb− | (1) |
| H2O2 + hvb+ → ˙OOH + H+ | (2) |
| H2O2 + ecb– → ˙OH + OH− | (3) |
| ˙OOH → ˙O2− + H+ | (4) |
From the above photocatalytic results, it can be found that the formation of the nanotwinned building blocks may be the reason for the improvement in the photocatalytic activity, and the photocatalytic capacity of the polycrystalline polyhedral 26-facet Cu7S4 microcages with a larger amount of nanotwinned building blocks would be improved, because the nanotwins inside the Cu7S4 cages might keep the transport property of free charges as in perfect crystals, and could decrease the recombination of photoinduced electrons and holes, and increase the lifetime of the electron–hole pairs.20 Therefore, the photochemical activities of the above two samples can further demonstrate that a greater number of nanotwins can significantly prevent the recombination of photogenerated electron–hole pairs to improve their photocatalytic activities.
Conclusions
In summary, a facile synthesis of nanotwinned polyhedral 26-facet Cu7S4 microcages enclosed within polycrystalline shells was successfully achieved via a glycol-assisted Cu2O-templated growth/etching route. The formation of crystallinity-controllable shells can be attributed to the aggregation behavior of nanoplate building blocks in solvents of different polarity. The photocatalytic superiority of these as-prepared polycrystalline cages can be attributed to the presence of a larger number of active components of nanotwinned building blocks, which exhibited higher adsorption ability and catalytic activity for enhancing photocatalytic degradation of MB dye.Acknowledgements
We thank the support from the National Science Foundation of China (NSFC No. 51071116), National Basic Research Program of China (No. 2010CB635101) and the Natural Science Foundation of Shaanxi Province (No. 2011JZ008).Notes and references
- S. H. Jiao, L. F. Xu, K. Jiang and D. S. Xu, Adv. Mater., 2006, 18, 1174 CrossRef CAS.
- W. X. Zhang, Z. X. Chen and Z. H. Yang, Phys. Chem. Chem. Phys., 2009, 11, 6263 RSC.
- H. L. Cao, X. F. Qian, C. Wang, X. D. Ma, J. Yin and Z. K. Zhu, J. Am. Chem. Soc., 2005, 127, 16024 CrossRef CAS.
- Z. Y. Wang, D. Y. Luan, C. M. Li, F. B. Su, S. Madhavi, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 16271 CrossRef CAS.
- Y. Qin, R. C. Che, C. Y. Liang, J. Zhang and Z. W. Wen, J. Mater. Chem., 2011, 21, 3960 RSC.
- H. G. Zhang, Q. S. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766 CrossRef CAS.
- F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef CAS.
- S. J. Ding, J. S. Chen, G. G. Qi, X. N. Duan, Z. Y. Wang, E. P. Giannelis, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 21 CrossRef CAS.
- M. S. Yavuz, Y. Y. Cheng, J. Y. Chen, C. M. Cobley, Q. Zhang, M. Rycenga, J. W. Xie, C. Kim, K. H. Song, A. G. Schwartz, L. V. Wang and Y. N. Xia, Nat. Mater., 2009, 8, 935 CrossRef CAS.
- L. S. Yu, Y. Y. Lv, G. D. Chen, X. L. Zhang, Y. W. Zeng, H. Y. Huang and Y. Y. Feng, Inorg. Chim. Acta, 2011, 376, 659 CrossRef CAS.
- S. D. Sun, X. P. Song, C. C. Kong, S. H. Liang, B. J. Ding and Z. M. Yang, CrystEngComm, 2011, 13, 6200 RSC.
- P. Roy, K. Mondal and S. K. Srivastava, Cryst. Growth Des., 2008, 8, 1530 CAS.
- C. Y. Wu, S. H. Yu, S. F. Chen, G. N. Liu and B. H. Liu, J. Mater. Chem., 2006, 16, 3326 RSC.
- Z. Fang, X. Y. Wang, J. M. Shen, X. Lin, Y. H. Ni and X. W. Wei, Cryst. Growth Des., 2010, 10, 469 CAS.
- C. X. Lai, Q. B. Wu, J. Chen, L. S. Wen and S. Ren, Nanotechnology, 2010, 21, 215602 CrossRef.
- X. P. Feng, Y. X. Li, H. B. Liu, Y. L. Li, S. Cui, N. Wang, L. Jiang, X. F. Liu and M. J. Yuan, Nanotechnology, 2007, 18, 145706 CrossRef.
- D. F. Zhang, H. Zhang, Y. Shang and L. Guo, Cryst. Growth Des., 2011, 11, 3748 CAS.
- H. L. Xu, W. Z. Wang, W. Zhu and L. Zhou, Nanotechnology, 2006, 17, 3649 CrossRef CAS.
- S. D. Sun, D. C. Deng, C. C. Kong, X. P. Song and Z. M. Yang, Dalton Trans., 2012, 41, 3214 RSC.
- M. C. Liu, L. Z. Wang, G. Q. Lu, X. D. Yao and L. J. Guo, Energy Environ. Sci., 2011, 4, 1372 CAS.
- K. V. Singh, A. A. M. Morales, G. T. S. Andavan, K. N. Bozhilov and M. Ozkan, Chem. Mater., 2007, 19, 2446 CrossRef CAS.
- P. Roy, K. Mondal and S. K. Srivastava, Cryst. Growth Des., 2008, 8, 1530 CAS.
- J. Zhou, G. L. Zhao, B. Song and G. R. Han, CrystEngComm, 2011, 13, 2294 RSC.
- Q. H. Mu, Y. G. Li, H. Z. Wang and Q. H. Zhang, CrystEngComm, 2011, 13, 6258 RSC.
- J. Shi, J. Li, X. J. Huang and Y. W. Tan, Nano Res., 2011, 4, 488 Search PubMed.
- Z. H. Wang, S. P. Zhao, S. Y. Zhu, Y. L. Sun and M. Fang, CrystEngComm, 2011, 13, 2262 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20107k |
| This journal is © The Royal Society of Chemistry 2012 |
