Exploiting duality in nature: industrial examples of enzymatic oxidation and reduction reactions
K.
Robins
and
A.
Osorio-Lozada
Lonza AG–Nutrition R&D (LCRDN), Visp Wallis 3930, Switzerland. Tel: +41279486932
First published on 12th April 2012
Abstract
Where there is oxidation there will always be reduction. Oxidation was coined by Morveau and Lavoisier in 1790 from the French ox(ygène) and (ac)die and reduction was coined around the same time from “re” meaning “back” and “ducere” meaning to “bring back” in the sense of lower, diminish or lessen (Online Etymology Dictionary, http://www.etymonline.com). In this sense an oxidizing agent gives oxygen to another substance or removes hydrogen (loss of electron) from it while a reducing agent carries out the opposite reaction. Industry is increasingly exploiting this duality for the production of industrially relevant intermediates for active pharmaceutical ingredients (APIs). In some cases the oxidized product is of interest and sometimes the reduced product. These enzymatic reactions are carried out under mild conditions with often high regio- or enantioselectivity compared to their chemical counterparts and in the case of enzymatic oxidation over-oxidation can be avoided in most cases. The key to the successful use of an enzymatic oxidation or reduction reaction in large scale is the regeneration of the redox partner in order to reach economic targets for the process.
 K. Robins | Karen Robins (born 1959) completed her Bachelor of Science with honours in Microbiology at the University of Sydney in 1980 and her Masters Degree in Applied Sciences (Biopharmaceuticals) at the University of NSW in 2001. She joined the Lonza Biotechnology group in 1984. She also spent 4.5 years in Lonza Guangzhou, PRC during the building and start-up of the nicotinamide plant. In this time she worked on the second generation biocatalyst for the nicotinamide process. Currently she is a project leader and senior research associate in the Biotransformation & Route Selection R&D Group, Nutrition of Lonza Ltd. The scope of her work includes the use of biotransformations and fermentation technologies for the production of nutritional products, agrochemicals and small molecules including the production of secondary metabolites for cytostatic and cytotoxic application. |
 A. Osorio-Lozada | Antonio Osorio-Lozada received his B. Sc. in Pharmaceutical Chemistry from The National University of San Marcos (Lima, Peru). He worked for three years in DSM-Anti-infectives division in Peru (Sinquisa) and two years in dairy food industry (Gloria S. A.) in fermentation and quality control departments. He obtained his PhD in Medicinal and Natural Products Chemistry from The University of Iowa under the guidance of Horacio F. Olivo. His research focused in biocatalysis; asymmetric sulfoxidations and hydroxylations and in synthetic organic chemistry; aldol reaction and γ-ylidenetetronates. Antonio is currently project leader in Biotransformation & Route Selection in Lonza Ltd, Visp. His activities focused in search for enzymatic activities from enriched environmental samples, cloning end expression of enzymes, fermentation of secondary metabolites and synthesis of the required substrates for a desired biocatalytic step. |
Introduction
There are many examples of enzymatic oxidation and reduction reaction products in industry (see Fig. 1). In recent years enzymatic reductions in the form of enantioselective ketoreductases have moved from the realm of interesting laboratory scale reactions to the method of choice for large scale asymmetric reductions. One of the most well-known industrial processes is the two step enzymatic process for the production of ethyl (R)-4-cyano-3-hydroxybutyrate, an important intermediate in atorvastatin production.2,3 The first step of this synthesis is the enzymatic reduction of ethyl 4-chloroacetoacetate with an enantioselective ketoreductase to produce ethyl (S)-4-chloro-3-hydroxybutanoate. Other enzymatic reductions like enoate or ene reductases are also emerging as interesting reactions for industry. One example is the production of 6-aminocaproic acid by the enzymatic reduction of 6-aminohex-2-enoic acid.4 In the case of oxidation there are a few reactions that have been used for a long time in industry. One example is glucose oxidase, which oxidizes D-glucose to D-gluconolactone and hydrogen peroxide. This enzyme is an important industrial enzyme used for its oxidizing properties in food preservation and also in strengthening bread matrices.5 Other more recent enzymatic oxidations processes that are being developed by industry are the use of L-iso-leucine dioxygenase for the production of (2S, 3R, 4S)-4-hydroxyleucine6 and the use of monoamine oxidase for the production of the cyclic imine, a key intermediate in the synthesis of telapravir.7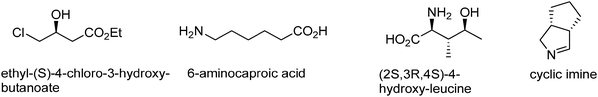 | ||
| Fig. 1 Interesting products of enzymatic oxidation and reduction reactions. | ||
Lonza has exploited this duality over the years by developing enzymatic oxidation or reduction platforms that can be used for various product categories. Lonza has looked at numerous ketoreductions using commercial enzymes. However, this article will concentrate on other examples of enzymatic oxidation and reduction like aromatic hyroxylations, ethyl and methyl group oxidation, oxidation using Gluconobacter oxydans (polyol dehydrogenases) and an example of reduction using an enoate reductase. All of these processes have been implemented by Lonza as whole cell processes.
Oxidation
Lonza processes for the regiospecific oxidation of meso-erythritol for the production of L- erythrulose, the hydroxylation of 4-hydroxyphenylacetic acid to produce 3,4-dihydroxyphenylacetic acid and the regioselective oxidation of the methyl group of 2,5-dimethylpyrazine for the production of 5-methylpyrazine-2-carboxylic acid will be described in the following text in more detail.L-Erythrulose
Gluconobacter oxydans specializes in growing in low pH, sugar rich environments like flowers, fruit, alcoholic beverages or soft drinks.8 It oxidizes a wide range of alcohols and carbohydrates albeit incompletely. These strains are used for the industrial production of L-sorbose which is used in vitamin C synthesis,9 6-amino-L-sorbose for Miglitol, an anti-diabetic drug and the tanning agent dihydroxyacetone just to name a few applications.10 The group of Deppenmeier11 have sequenced the genome of Gluconobacter oxydans 621H and identified approximately 75 putative oxido-reductases (dehydrogenases). A large proportion of these dehydrogenases are membrane bound and directly associated with the electron transport chain. The rest are found in the cytoplasm. They are either NAD(P)+ or PQQ (pyrroloquinoline quinone) dependent. This organism is an excellent candidate for producing enantiomerically pure hydroxyacids, hydroxyketones and alcohols for nutrition products and API intermediates and avoids the environmentally unfriendly protecting group strategy that would be required to carry out the same syntheses with classical chemistry.Lonza has several large scale processes that use Gluconobacter oxydans polyol dehydrogenases. One is the production of the keto-sugar, L-erythrulose.12L-Erythrulose is used as an artificial skin tanning agent. It diffuses more uniformly into the skin than the other well-known tanning agent, dihydroxyacetone which can also be produced by the enzymatic oxidation of glycerol. In this process meso-erythritol is oxidized to L-erythrulose by Gluconobacter oxydans DSM 13061, formerly known as Acetobacter oxydans (see Fig. 2).
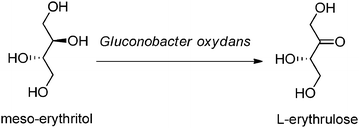 | ||
| Fig. 2 Enzymatic oxidation of meso-erythritol. | ||
This reaction was already known in the seventies when this oxidation was carried out by Acetobacter suboxydans ATCC 62113 but the yield was only 45–50% and after product isolation using column chromatography the isolated yield was only 23%. Lonza improved this process to achieve almost quantitative yields of L-erythrulose from meso-erythritol. During media optimization studies it was shown that the best inducers of the membrane bound polyol dehydrogenase14 were mannitol and glycerol. The optimization studies resulted in shortened process times and higher biotransformation rates. The fed-batch process (see Fig. 4) was carried out in the following manner: The pre-culture involved a two-step seed. The first seed (500 mL) was carried out in a shaker flask culture at 28 °C for 18 h. This culture was used to inoculate the second seed in a 10 L culture (20 L fermenter) also at 28 °C for 14 h. The 450 L fermenter containing 260 L of medium was then inoculated with the second seed (3.8% inoculum). The fermentation was run at 28 °C with an initial pH of 6.9. The pH decreased during growth and once the pH had reached pH 4.6 it was maintained at this value. Another important parameter was the partial pressure of oxygen which was controlled at >50%. The meso-erythritol was dosed until an end concentration of 140 g L−1L-erythrulose had been reached in the fermenter. This corresponded to a 97% non-isolated conversion yield. The fermentation time was about 55 h. During the process the L-erythrulose concentration was monitored spectrophotometrically using a wavelength of 277 nm and as can be seen in Fig. 3 the L-erythrulose production occurs in parallel to growth of the culture.
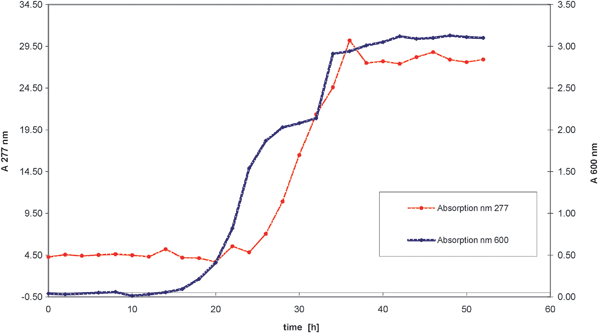 | ||
| Fig. 3 Growth and L-erythrulose production during the fermentation (L-erythrulose concentration and growth measured at 277 nm and 600 nm, respectively). | ||
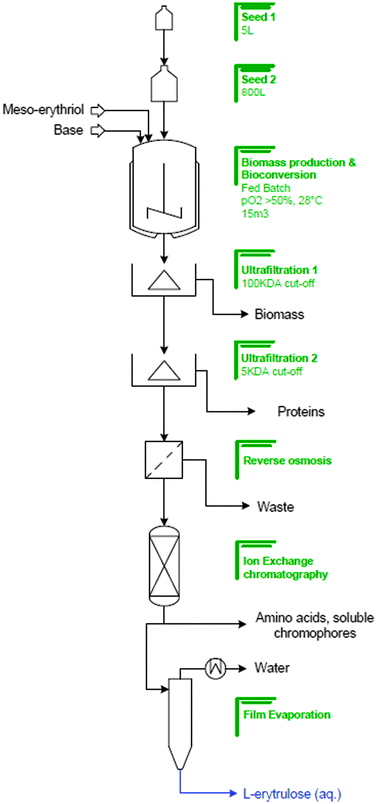 | ||
| Fig. 4 Production of L-erythrulose. | ||
The DSP was also improved to give an isolated yield between 80–90%. The first step of the product isolation was the removal of biomass and protein from the solution using two ultrafiltration steps. The first step used a membrane with a molecular cut-off of 100 kDa and the second with a cut-off of 5 kDa. This was followed by reverse osmosis to concentrate the solution and then ion exchange chromatography which removed residual amino acids and soluble chromophores from the solution. The last step was the concentration of the solution by film evaporation. This process was carried out in 15 m3 with an overall molar yield of 85–90% (w/w) based on the substrate, meso-erythritol.
Lonza and the group of Molinari also examined the production (S)-2-(hydroxy)methyl-propionic acid by oxidizing 2-methyl-1,3-propandiol with Acetobacter pasteurianus. The enantiomeric excess achieved using this system was 96–97%.15 This process, however, was not scaled.
3,4-Dihydroxyphenylacetic acid (3,4-DHPA)
One of the most commonly used reactions to increase functionality is the insertion of oxygen into organic molecules, for example, the hydroxylation of an aromatic ring and alkyl group oxidation.The exploitation of these hydroxylation reactions has been accomplished with chemical technologies using hydrogen peroxide with iron16 or copper17 and aluminum complexes18 hydroxylation of naphthalene derivatives with cumene hydroperoxide19 and more recently with the use of irradiation and anthraquinone as oxygen sensitizer.20 Although these chemical reactions are well established in the industry, the reaction conditions are harsh and the waste management makes up most of the production costs rendering them exceedingly environmentally unfriendly. Aromatic hydroxylation is a reaction performed in most biological systems. The enzyme systems responsible for this reaction are monooxygenases, dioxygenases, flavin monooxygenases, peroxidases, and phenol hydroxylases21
Lonza has an enzymatic hydroxylation platform for the hydroxylation of heterocycles which was used for the large scale production of 6-hydroxynicotinic acid and 5-hydroxypyrazine carboxylic acid and also the enzymatic hydroxylation system, analogous to β-oxidation of fatty acids, which has been used for the industrial production of L-carnitine.22 The next example illustrates Lonza's continuous expansion of its hydroxylation platform to include phenyl group hydroxylation with the hydroxylation of 4-hydroxyphenylacetic acid (4-HPA). Approximately 400 strains from the Lonza's aromatic-hydroxylation-focused strain collection were screened in high throughput (HTPS) format for this aromatic hydroxylation. The aerobic bacteria, Arthrobacter protophormiae KIE74 was one of the best candidates in terms of selectivity and kinetic conversion of 4-HPA to 3,4-DHPA (see Fig. 5). This particular microbial isolate exhibited doubling time of 75 min. Medium optimization in shaker flasks allowed quick transfer to fermentation experiments.
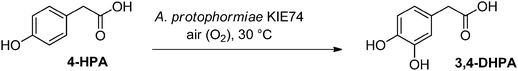 | ||
| Fig. 5 Hydroxylation of 4-HPA. | ||
Several fermentations were performed to explore pH, optimal stirring rate, oxygen dissolution, inoculum size and substrate load. Typical fermentation was performed at pH 7.0, 30 °C, with a stirring range of 300–500 rpm, air flow rate from 0.3 to 0.5 L.min-1. A 2 L fermenter was inoculated with 0.1 L of 16 h old pre-culture (1% inoculum). After 5 h 20 g of 4-HPA (solid) was added directly to the fermenter and after 6 h a second portion of 40 g of 4-HPA was added. The reaction was completed after approximately 19 h making a total time of 24 h (see Fig. 6). The fermented broth was filtered and the filtrate was adjusted to pH 3 and extracted with ethyl acetate (5 × 200 mL). The combined organic layers were evaporated to give a light-brown solid (52% yield). NMR analysis of the crude product indicated that only the desired product, 3,4-DHPA was present and no side products which indicate the high selectivity of this hydroxylation reaction.
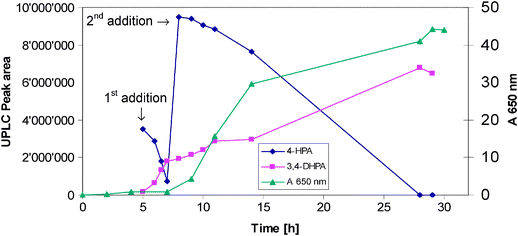 | ||
| Fig. 6 Production of 3,4-DHPA (growth measured at 650 nm). | ||
Methyl group oxidation
Carboxylic acids are versatile functional groups. A common synthetic route for the preparation of aromatic carboxylic acids is via the Kolbe-Schmidt reaction which has strong substrate preference for phenols. A more versatile approach is the oxidation of methyl group in aromatic systems. Different methods are used such selenium dioxide23 and an industrial relevant example is the preparation of terephthalic and isophthalic acid by oxidation of p-xylene and m-xylene, respectively, in acetic acid at 175–225 °C.24 Methods using biocatalysts have the advantage of providing a mild and selective methyl group oxidation. An early report from Kitagawa indicated that toluene adapted cells of Pseudomonas aeruginosa oxidized toluene to benzoic acid.25 The Rhodococcus genus has also proven their versatility for oxidation of o-xylene to 3-methyl catechol,26 and Sphingomonas paucimobilis is capable of oxidizing 2,6-dimethylnaphthalene to 6-methyl-2-naphthoic acid.275-methylpyrazine-2-carboxylic acid
Lonza's platform for methyl- and ethyl group oxidation involves the use of Pseudomonas putida ATCC 33015 for the regiospecific oxidation of methyl group in heterocycles to the corresponding carboxylic acids and the use of Pseudomonas oleovorans ATCC 29347 for the oxidation of heterocycles substituted with an ethyl group to the corresponding acetic acid derivative.28 The complete chemo selectivity of these reactions is shown, for example, by the oxidation of the ethyl group of 3-ethyl-5-methylpyridine to produce (5-methyl-pyridyl-) acetic acid exclusively.29 An illustrative example of the methyl group oxidation is the preparation of 5-methylpyrazine-2-carboxylic acid (MPCA) from 2,5-dimethylpyrazine (DMP) (see Fig. 7). MPCA is a versatile intermediate for the preparation of active pharmaceutical ingredients and agrochemicals. | ||
| Fig. 7 Selective oxidation of 2,5-DMP for the preparation of MPCA. | ||
P. putida ATCC 33015 cells were grown on xylenes as a sole source of carbon and energy as illustrated in Fig. 8. High concentrations are toxic to the cells and it is therefore important to control the concentration of the xylene at low levels. This measurement is carried out with on-line UV measurements or on-line HPLC. DMP is also toxic to the cells and its addition to the fermenter is also controlled so that the concentration remains below 2 g L−1 allowing a logarithmic cell growth during the reaction time. In large scale (15 m3) a mixture of xylene and DMP was added and a maximum concentration of 24 g L−1 MPCA was reached with more than 95% purity. At the end of the fermentation MPCA is the only oxidation product observed and only 0.1% DMP remains. The product was recovered from the fermentation broth by adjusting the pH to 3 followed by centrifugation.
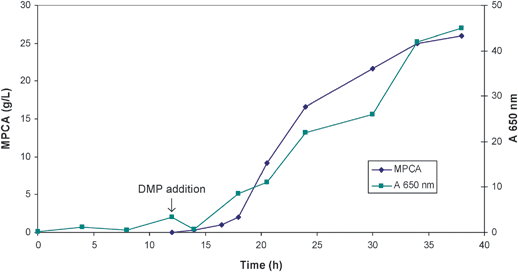 | ||
| Fig. 8 Production of MPCA by oxidation on DMP (growth measured at 650 nm). | ||
Reduction
The main enzymatic reduction reactions of interest that are used by industry today are the NAD(P)H dependent keto-, ene- and enoate reductions. Lonza developed a whole cell process for the production of (R)-2-hydroxymethyl hexanoic acid ethyl ester which will be described here in more detail.(R)-2-hydroxymethyl hexanoic acid ethyl ester (HHAEE)
The production of (R)-2-hydroxymethyl propionic acid ethyl ester by reduction of the 2-formyl propionic acid was already reported in the literature in 1983.30 A yield of 70–80% and an enantiomeric excess (ee) of 60–65% was achieved. Also Matzinger and Leuenberger31 showed that Streptomyces hydrogenans and Nocardia opaca could produce (R)-2-hydroxymethyl pentanoic acid ethyl ester with yields >50% and ee = 96.5% and yields of 50% and ee = 87%, respectively.Several possible routes were evaluated on paper including several routes that started with the Lonza building block, malondinitrile for the chemical synthesis of the dinitrile starting material for the enzymatic synthesis. The subsequent regiospecific enzymatic hydrolysis of one of the nitrile groups followed by esterification and chemical reduction proved to be step intensive which translates into high costs.
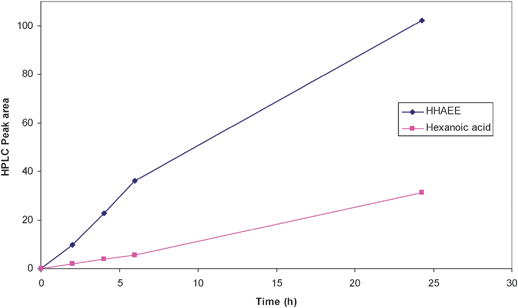
The 2-formylhexanoic acid ester exists as a tautomer which means that theoretically a yield of 100% could be achieved making it economically attractive as a method of synthesis (see Fig. 9). This strategy was used for the asymmetric synthesis via enzymatic reduction to produce the (R)-hydroxymethyl hexanoic acid ethyl ester.32 The Lonza strain collection was screened for microorganisms with an enoate reductase capable of reducing this compound. A total of only 67 strains from the Lonza strain library were screened and 5 strains were found capable of reducing 2-formylhexanoic acid ester. The baker's yeast as expected reduced the compound but the enantioselectivity was very low (63%). The three other yeasts capable of reducing the substrate showed either low enantioselectivity or low yield (Candida magnoliae DSM 70638 ee = 33%, Hansenula anomala DSM 70260 ee = 69% and Torulopsis petrophilum ATCC 20225 ee >99% but the yields were extremely low). However one strain, Rhodococcus rhodochrous NCIMB 11216 was able to produce the desired product with high yield and high enantioselectivity. Due to the tight timelines set by the customer a non-optimized process was used to produce gram amounts of the desired product. The strain was fermented in batch mode to produce the active biomass. A soya peptone, yeast extract and sucrose medium was used and the fermentation was carried out at 28 °C and at pH 7.0. The biotransformation was also carried out in phosphate buffer at the same pH and temperature as the fermentation. After a reaction time of 24 h the 2-formyl hexanoic acid ester was converted to the hydroxymethyl hexanoic acid ester and one major sideproduct, hexanoic acid (see Fig. 10).
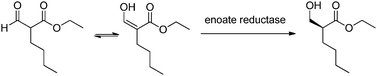 | ||
| Fig. 9 Enzymatic reduction of 2-formylhexanoic acid. | ||
 | ||
| Fig. 10 Production of (R)-2-hydroxymethyl hexanoic acid ethyl ester. | ||
After the biotransformation was completed the biomass was separated by centrifugation and the product was isolated from the bio-solution (see Table 1).
| Solution | Step | Product Solution | Product Assay (quant. NMR) |
|---|---|---|---|
| Biosolution (6.95 L) | Extraction (4.0 L ethyl acetate) | ||
| Organic phase | Concentrated to dryness | Yellow-brown liquid (8.8 g) | 75–80% |
| Oily residue | Dissolved in 90 ml ethyl acetate, washed with 10% CaCO3 (30 ml × 30 min) | ||
| Organic phase | Dried NaSO4, filtered, concentrated to dryness | Yellow–brown liquid (7.8 g) | 85–90% |
| Oily residue | Distilled under reduced pressure | Colourless liquid (6.0 g) | 93.7% |
The bio-solution was extracted with ethyl acetate and concentrated to dryness. The oily residue was dissolved in ethyl acetate and washed with a 1% solution of CaCO3 to remove the hexanoic acid and then dried with NaSO4, filtered and concentrated to dryness. The oily residue was then destilled under reduced pressure (5 mbar at 105–110 °C). The isolated yield was 55% with an ee >95% and a purity of 93.7% (see Table 2).
Several aspects of this process need to be optimized before it could be implemented in large scale. The reductase should be cloned and over-expressed in E. coli. The enzymatic activity responsible for the formation of the hexanoic acid needs to be inactivated (or absent) in the new host so that the side product concentration can be reduced and the yield increased. The amount of solvent used in the DSP should be reduced and the use of continuous extraction and reverse osmosis should be evaluated.
Future
Enzymatic oxidation and reduction are already important reactions for the large scale production of interesting API intermediates. These reactions will, however, become increasingly important for industry as the use of hazardous reagents and harsh conditions become more regulated and environmental consideration become more important. The large palette of available oxidation reactions offer a biocatalytic tool box with high stereo-, regio- and chemoselectivity which can be carried out under mild conditions and avoid the harsh conditions that typically characterize the chemical methods. Stability and productivity, however, need to be improved in some cases, especially for the P450 cytochrome monooxygenases. In the case of enzymatic reduction ketoreductases are already established as the tool of choice for asymmetric reduction of ketone groups and are replacing the mature chemical route via asymmetric hydrogenation. Enoate and ene-reductases are beginning to emerge as useful tools for the reduction of the double bonds of α, β-unsaturated aldehydes and carboxylic acids. In addition to these reductions the non-NAD(P)H dependent reductases that are often found in anaerobic systems deserve more attention. These enzymes can use electrons channeled from hydrogen gas, carbon monoxide, formate or even from an electrochemical source via artificial electron mediators like reduced viologen derivatives. There are indications that these enzymes may in some cases have higher productivity and expand the substrate spectrum available for reduction.33Acknowledgements
We would like to thank Christoph Niederhauser for drawing the diagram of the erythrulose process for this article and Livia Artuso for providing the data for Fig. 3.References
- Online Etymology Dictionary, http://www. etymonline.com.
- S. K. Ma, J. Gruber, C. Davis, L. Newman, D. Gray, A. Wang, J. Grate, G. W. Huisman and R. A. Sheldon, Green Chem., 2010, 12, 81–86 RSC.
- N. Kizaki, Y. Yasohara, J. Hasegawa, M. Wada, M. Kataoka and S. Shimizu, Appl. Microbiol. Biotechnol., 2001, 55, 590–595 CrossRef CAS.
- US Pat., 7 491 520, 2009 Search PubMed.
- A. Bonet, C. M. Rosell, P. A. Caballero, M. Gomez, I. Perez-Munuera and M. A. Lluch, Food Chem., 2006, 99(2), 408–415 CrossRef CAS.
- M. Hibi, T. Kawashima, T. Kodera, S. V. Smirnov, P. M. Sokolov, M. Sugiyama, S. Shimizu, K. Yokozeki and J. Ogawa, Appl. Environ. Microbiol., 2011, 77(19), 6926–6930 CrossRef CAS.
- A. Znabet, M. Polak, E. Janssen, F. J. de Kanter, N. Turner, R. V. Orru and A. Ruijter, Chem. Commun., 2010, 46, 7918–7920 RSC.
- Bergey's Manual of Systematic Bacteriology, ed. N. R. Krieg and J. G Holt, William & Wilkins, Baltimore, 1st edn 1984, vol. 1, section 4, pp. 275 Search PubMed.
- J. Boudrant, Enzyme Microb. Technol., 1990, 12, 322–329 CrossRef CAS.
- U. Deppenmeier, M. Hoffmeister and C. Prust, Appl. Microbiol. Biotechnol., 2002, 60, 233–242 CrossRef CAS.
- C. Prust, M. Hoffmeister, H. Leisegang, A. Wiezer, W. F. Fricke, A. Ehrenreich, G. Gottschalk and U. Deppenmeier, Nat. Biotechnol., 2005, 23(2), 195–200 CrossRef CAS.
- Worldwide WO 0 142 483 A1, 2001.
- H. J. Haas and B. Matz, Liebigs Ann. Chem., 1974,(2), 342–344 CrossRef CAS.
- J. Voss, A. Ehrenreich and W. Leibl, Microbiology, 2010, 156(6), 1890–1899 CrossRef CAS.
- F. Molinari, R. Gandolfi, R. Villa, E. Urban and A. Kiener, Tetrahedron: Asymmetry, 2003, 14, 2041–2043 CrossRef CAS.
- G. A. Hamilton, J. P. Friedman and P. M. Campbell, J. Am. Chem. Soc., 1966, 88, 5266–5268 CrossRef CAS.
- E. A. Karakhanov, A. L. Maximov, Y. S. Kardasheva, A. A. Skorkin, S. V. Kardashev, E. A. Ivanova, E. Lurie-Luke, J. A. Seeky and S. L. Cron, Ind. Eng. Chem. Res., 2010, 49, 4607–4613 CrossRef CAS.
- M. E. Kurz and G. J. Johnson, J. Org. Chem., 1971, 36, 3184–3187 CrossRef CAS.
- L. Zhu and L.-H. Zhang, Tetrahedron Lett., 2000, 41, 3519–3522 CrossRef CAS.
- N. Tada, K. Hattori, T. Nobuta, T. Miura and A. Itoh, Green Chem., 2011, 13, 1669–1671 RSC.
- P. Di Gennaro, A. Bargna and G. Sello, Appl. Microbiol. Biotechnol., 2011, 90, 1817–1827 CrossRef CAS.
- H. G. Kulla, Chimia, 1991, 45, 81–85 CAS.
- C. H. Fisher, J. Am. Chem. Soc., 1934, 56, 2056–2057 CrossRef CAS.
- R. J. Sheenan, Ullman Encyclopedia Chemical Technology.
- M. Kitagawa, J. Biochem., 1956, 43, 553–563 CAS.
- S. R. Bickerdike, R. A. Holt and G. M. Stephens, Microbiology, 1997, 143, 2321–2329 CrossRef CAS.
- T. K. Dutta, S. A. Selifonov and I. Gunsalus, Appl. Environ. Microbiol., 1998, 64, 1884–1889 CAS.
- A. Kiener, Angew. Chem., Int. Ed. Engl., 1992, 3(6), 774–775 CrossRef.
- A. Kiener, Chemtech, 1995, 25, 31–35 CAS.
- M. R. Züger, F. Giovanni and D. Seebach, Angew. Chem., 1983, 95(12), 1024 CrossRef.
- P. K. Matzinger and G. W. Leuenberger, Appl. Microbiol. Biotechnol., 1985, 22, 208–210 CrossRef CAS.
- K. T. Robins presented at the 11th Swiss-Japanese Joint Meeting on Biotechnology and Bioprocess Development, Minusio, Switzerland October, 2008.
- H. Simon, J. Bader, H. Günther, S. Neumann and J. Thanos, Angew. Chem., Int. Ed. Engl., 1985, 24, 539–553 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
