Mesoporous Zr-SBA-15 as a green solid acid catalyst for the Prins reaction†
Dong Minh
Do
,
Stephan
Jaenicke
and
Gaik-Khuan
Chuah
*
Department of Chemistry, National University of Singapore, 3 Science Drive, Singapore 117543. E-mail: chmcgk@nus.edu.sg; Fax: 65 67791691; Tel: 65 6516 2918
First published on 27th March 2012
Abstract
Mesoporous Zr-SBA-15 platelets were prepared with different pore dimensions from 4 to 8 nm by the simple procedure of varying the amount of water in the synthesis gel. Narrow pore size distributions were obtained for water–tetraethoxysilane ratios between 208 and 639, but samples formed using a lower ratio of 100 had a broad pore size distribution. Thermogravimetric measurements showed that the interaction between the Pluronic template and the inorganic framework was affected by the amount of water in the synthesis gel. More zirconium was incorporated into the silica framework when the synthesis was conducted in a more dilute system. The Zr-SBA-15 obtained from this synthesis forms platelets with relatively short channels. The catalytic activity was tested for C–C-coupling (Prins reaction). The terpene alcohol Nopol, the product of an intermolecular Prins reaction between β-pinene and paraformaldehyde, could be obtained with excellent selectivity. The mesoporous structure of the catalyst together with the presence of zirconium in the silica framework, which confers strong Lewis acidity as well as weak Brønsted acidic sites, are essential for the activity and selectivity of the reaction.
Introduction
Acid-catalyzed reactions are important in petrochemical and fine chemical synthesis.1 Using solid acids instead of mineral acids offers green synthetic routes to the desired chemicals, due to ease of product isolation, minimization of waste in work-up and reuse of catalysts. Hence, efficient and selective solid acids are desired. An example of an acid-catalyzed C–C coupling reaction is the Prins reaction where aldehydes are added to alkenes.2 Different products such as 1,3-diols, 1,3-dioxanes or unsaturated alcohols can be formed, depending on the reaction conditions.3,4 Various acids have been reported such as hydrochloric acid, alkyl aluminium chlorides,5,6 stannic chloride,7–9 indium chloride in ionic liquids,10 and heteropolyacids.11 The Prins reaction of β-pinene with paraformaldehyde is used in the synthesis of Nopol, a bicyclic unsaturated primary alcohol (Scheme 1). Its ester with acetic acid, nopyl acetate, is an artificial fragrance compound which is found in the formulations of many household products such as pesticides, detergents, soaps and polishes.12 Normally, Nopol is synthesized in homogeneous systems using either ZnCl2 or acetic acid as the catalyst, or by autoclaving the mixture of formaldehyde and β-pinene for several hours at 150–230 °C.13 As tin was found to be active for the homogeneously-catalyzed reaction,7–9 various supported catalysts have been prepared by loading this metal onto high surface area supports like MCM-41, SBA-15 or kenyaite (a sodium silicate) through ion exchange, incipient wetness impregnation and chemical vapor deposition14–17 and by incorporation into the silica framework of mesoporous SBA-15.18 In particular, Corma and Renz19 reported that Sn-MCM-41 was very active in the Prins reaction of β-pinene with paraformaldehyde, forming Nopol with a 94% selectivity at 93% conversion. The use of butylnitrile instead of toluene as a solvent improved the selectivity by moderating the strength of the acidic sites at the catalyst. | ||
| Scheme 1 Prins condensation of β-pinene and paraformaldehyde. | ||
Non-tin-containing heterogeneous catalysts reported for Nopol synthesis include mesoporous iron phosphate,20 mesoporous Zn-Al-MCM-41,21 ZnCl2 impregnated Indian Montmorillonite,22 and Fe–Zn double cyanide.23 However, mesoporous iron phosphate gave only Nopol yields of 2–85% at a catalyst to substrate ratio of 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mmol.20 An even higher catalyst to substrate molar ratio (6.6
5 mmol.20 An even higher catalyst to substrate molar ratio (6.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) was required to reach 100% conversion. Similarly, the use of Al-MCM-41 and Zn-Al-MCM-41 gave Nopol yields of 27–84% but at a catalyst
5) was required to reach 100% conversion. Similarly, the use of Al-MCM-41 and Zn-Al-MCM-41 gave Nopol yields of 27–84% but at a catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate ratio of 0.2 g (∼ 3.3 mmol)
substrate ratio of 0.2 g (∼ 3.3 mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 mmol.21
0.75 mmol.21
We have found that hydrous zirconia and Zr-zeolite beta are good catalysts for the intramolecular Prins cyclisation of citronellal to isopulegols.24,25 These catalysts possess strong Lewis acid sites and weak Brønsted sites, both of which are essential for the reaction.24 Further studies using mesoporous TUD-1 with framework incorporation of Al and Zr showed the presence of synergy between the Brønsted and Lewis acid sites when applied as catalysts in the cyclisation of citronellal.26 However, when used in the more challenging intermolecular Prins reaction between β-pinene and paraformaldehyde, no synergy was observed.27 To investigate if there are other suitable catalysts besides the tin-based ones for intermolecular Prins reactions, we focused on zirconium-containing materials. The bulky size of β-pinene necessitates the use of mesoporous catalysts, as previous studies with this substrate have shown a higher reaction rate with Sn-MCM-41 than with microporous Sn-beta.19
Among the mesoporous materials, SBA-15 with tunable pore sizes of 4–10 nm arranged in a 2D-hexagonal p6mm structure has received much attention in the past decade because of its relatively large pore size and high hydrothermal stability in comparison with other mesoporous silica materials. While the synthesis of the pure silica mesoporous material is easily carried out in the presence of an appropriate surfactant, it has low catalytic activity due to the lack of acidic sites. The incorporation of other metal ions such as zirconium into the framework provides Lewis acidity. Mesoporous zirconium-doped silica is of interest due to the catalytic activity of the metal28–33 and the possibility of forming strong solid acids by sulfation.34–37 Recently, several novel routes have been developed for direct synthesis of Zr-SBA-15. For example, Newalkar et al.38 reported the direct synthesis of Zr-SBA-15 with Si/Zr of 10–80 using microwave irradiation. The materials formed had thicker walls than those formed by conventional synthesis.39 Materials with high zirconium content (Si/Zr 5–9) were prepared by Du et al.40 using urea as a pH adjustor. Cheng and coworkers41 showed that the synthesis of Zr-SBA-15 from the zirconium precursor ZrOCl2·8H2O was possible in the absence of HCl. However, the addition of HCl changed the morphology of the materials from rod-like structures to hexagonal platelets with short mesochannels of 150–350 nm in length, perpendicular to the hexagonal planes.42 Due to their structure, these platelet materials were less susceptible to diffusion limitations and pore blockage than conventional rod or fibre-like SBA-15. Hence, these materials are potentially useful catalysts in the intermolecular Prins reaction, where both the nature and strength of the acidic sites are essential for the formation of Nopol.43 In this study, we varied the water content in the synthesis gel and examined its effect on the pore size of the materials.
Experimental
Preparation of zirconium-incorporated SBA-15
First, 0.5 g Pluronic P123 was added to a solution containing 3.33 ml concentrated HCl (12 M) in water. After stirring the mixture for 4 h to obtain a clear solution, 1.15 ml tetraethyl orthosilicate (TEOS) and 0.162 g zirconium oxychloride (ZrOCl2·8H2O) were added. The mixture was stirred at 35 °C for 8 h (or 24 h) before hydrothermal treatment at 100 °C for 24 h in a Teflon-lined autoclave. The solid was recovered by filtration, washed, dried at 100 °C, and calcined at 550 °C for 8 h to remove the organic template. The amount of water was varied from 6.67 ml to 56.67 ml to give H2O/Si molar ratios of 100, 208, 423 and 639. The molar composition of the synthesis gel was 0.017 Pluronic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TEOS
1 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10 ZrOCl2·8H2O
0.10 ZrOCl2·8H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.77 HCl
7.77 HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–639 H2O. The samples are labeled as 10Zr-w-t where 10 denotes the Si/Zr ratio, w the H2O/Si ratio and t represents the aging time of the synthesis gel. A catalyst with a lower zirconium content, 50Zr-423-24, was also prepared.
100–639 H2O. The samples are labeled as 10Zr-w-t where 10 denotes the Si/Zr ratio, w the H2O/Si ratio and t represents the aging time of the synthesis gel. A catalyst with a lower zirconium content, 50Zr-423-24, was also prepared.
A procedure for Al-SBA-1544 was adapted for the preparation of non-platelet mesoporous zirconium silica (10ZrMPS, Si/Zr = 10). The molar composition of the gel was 0.016 Pluronic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TEOS
1 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10 ZrOCl2·8H2O
0.10 ZrOCl2·8H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.56 HCl
0.56 HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 H2O. A mixture of 2 g Pluronic P123 in 27.6 ml water was stirred at room temperature for 3 h before adding 2.4 ml of 5 M HCl, 4.82 ml TEOS and 0.696 g zirconium oxychloride. The solution was stirred for 4 h, hydrothermally treated in a Teflon-lined autoclave at 100 °C for 2 days before calcination at 500 °C for 8 h.
77 H2O. A mixture of 2 g Pluronic P123 in 27.6 ml water was stirred at room temperature for 3 h before adding 2.4 ml of 5 M HCl, 4.82 ml TEOS and 0.696 g zirconium oxychloride. The solution was stirred for 4 h, hydrothermally treated in a Teflon-lined autoclave at 100 °C for 2 days before calcination at 500 °C for 8 h.
Characterization of samples
The zirconium and silicon content of the samples was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) after dissolving a weighed amount in HF. X-ray diffraction measurements were carried out using a Siemens D5005 diffractometer equipped with a copper anode and variable slits. A step size of 0.004°/step and 0.02°/step was used for 2θ between 0.5–6° and 6–70°, respectively. The morphology of the samples was measured using a scanning electron microscope (JEOL JSM-5200). The UV-Vis absorption spectra were measured against a barium sulfate reference using a Shimadzu spectrophotometer (UV-2450) equipped with a diffuse-reflectance cell. 29Si MAS NMR spectra were measured on a Bruker DRX-400 wide-bore solid state spectrometer operating at a resonance frequency of 79.46 MHz with a spinning rate of 8 kHz, a pulse length of 4 μs and a recycle time of 20 s. 4 mm rotors were used and the 29Si chemical shifts are reported relative to tetramethylsilane. The interaction between Pluronic and the inorganic silica–zirconia was studied using thermogravimetric analysis-mass spectrometry (TGA-MS, MS-Pfeiffer Thermo Star) and simultaneous differential thermal analysis-thermogravimetric analysis (DTA-TGA, SDT 2960). Prior to the measurements, the as-synthesized sample was kept at 100 °C for 30 min before heating to 600 °C in air at a rate of 10 °C min−1. The evolution of water (m/z 18) and carbon dioxide (m/z 44) was monitored by the online mass spectrometer. The adsorption of β-pinene and formaldehyde on 10Zr-423-8 was investigated by DTA-TGA. The catalyst was first impregnated with β-pinene or formaldehyde in toluene and dried at room temperature under nitrogen before thermogravimetric analysis. A blank run was carried out using only the catalyst. The presence of residual products on the catalyst after the reaction was similarly studied. A heating rate of 10 °C min−1 in nitrogen from room temperature to 500 °C was used.The nitrogen adsorption–desorption isotherms were carried out using a Micromeritics Tristar 3000. Samples were degassed under nitrogen flow at 300 °C for 6 h before measurement. The surface area was calculated according to Brunauer–Emmett–Teller (BET) theory while the pore size distribution was obtained by the Barrett–Joyner–Halenda (BJH) method. The total amount of acidity was determined by temperature-programmed desorption (TPD) of ammonia. After pretreatment in helium (50 ml min−1) at 500 °C for 2 h, the sample was cooled to 150 °C, exposed to ammonia gas for 15 min and flushed with helium for another 2 h to remove any physisorbed ammonia. The temperature was then increased at a rate of 10 °C min−1 from 150 to 500 °C and the evolved gases were analyzed using an online mass spectrometer (Balzers Prisma 200). The nature of the acid sites was determined using infrared spectroscopy of adsorbed pyridine. A self-supporting wafer of the sample (8–10 mg) was mounted in a Pyrex IR cell with NaCl windows and degassed at 300 °C in a vacuum (10−3 mbar) for 2 h. After cooling to room temperature, pyridine was introduced into the cell at 22 mbar. Following evacuation for an hour, an IR measurement at 2 cm−1 and 64 scans was made using a Bio-Rad FTS 165 FT-IR spectrometer. Further measurements were made after the sample had been heated at 100 and 200 °C for 1 h at each temperature.
Catalytic studies
For catalytic testing, 0.156 ml (1 mmol) β-pinene, 0.0606 g (2 mmol) paraformaldehyde and 5 mL toluene were added into a 2-necked round-bottom-flask equipped with a reflux condenser. The reaction mixture was heated under stirring in an oil bath before adding 50 mg of catalyst. The reaction temperature was varied between 80–110 °C. Samples were withdrawn at regular time intervals and analyzed by gas chromatography. The products were identified by gas chromatography–mass spectrometry (GCMS). The reaction order in β-pinene and paraformaldehyde was studied at 100 °C by keeping one substrate concentration at 4 mmol and varying the other from 0.25 to 4 mmol in 15 mL toluene. To test for reuse, the catalyst was recovered by centrifugation after the reaction. Its activity for further batch reactions was tested following regeneration by either (i) washing with toluene or (ii) treatment with H2O2 solution at 40 °C overnight followed by drying at 100 °C.Results and discussion
Textural properties
Fig. 1 shows the low angle XRD patterns of calcined Zr-SBA-15. Except for 10Zr-100-24 prepared with the lowest H2O/Si ratio of 100, the other samples prepared with higher H2O content showed the typical (100), (110) and (200) diffraction peaks of the 2D-hexagonal p6mm structure. These diffraction peaks were already present after only 8 h aging time, although the intensity of the peaks was lower than in samples aged for 24 h. The peaks shifted to lower angles with increase of H2O in the synthesis gel, indicative of an increase in lattice spacing (Table 1). A longer aging time counter-acts this lattice expansion. It is possible that with longer aging time, syneresis takes place, resulting in a contraction of the inorganic framework. The wide-angle XRD spectra of the calcined samples (Fig. S1, ESI†) showed only a broad hump for 2θ between 15° to 35°, indicating the amorphous structure of the walls.42 No crystalline phase of zirconia was detected, suggesting that zirconium is either well-incorporated into the silica matrix or the zirconia crystallites are below the XRD detection limit of ∼4 nm. | ||
| Fig. 1 XRD patterns of calcined Zr-SBA-15 materials (a) 10Zr-100-24 (b) 10Zr-208-24 (c) 10Zr-208-8 (d) 10Zr-423-24 (e) 10Zr-423-8 and (f) 10Zr-639-24. | ||
| Sample | Surf. area /m2 g−1 | Pore vol. /cm3 g−1 | Pore diam. /nm | ao /nm | t/nm |
|---|---|---|---|---|---|
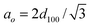 , t: wall thickness. , t: wall thickness. |
|||||
| 10Zr-100-24 | 544 | 0.79 | 5.6–7.3 | — | — |
| 10Zr-208-24 | 597 | 0.93 | 6.3 | 11.2 | 4.9 |
| 10Zr-423-24 | 627 | 1.09 | 7.4 | 11.3 | 3.9 |
| 10Zr-639-24 | 711 | 1.34 | 8.0 | 12.2 | 4.2 |
| 10Zr-208-8 | 621 | 0.93 | 6.0 | 12.0 | 6.0 |
| 10Zr-423-8 | 629 | 1.18 | 7.6 | 11.9 | 4.3 |
| 50Zr-423-24 | 909 | 1.31 | 6.3 | ||
| 10ZrMPS | 616 | 0.85 | 5.4 | — | — |
All samples have a high surface area (544–711 m2 g−1) and large total pore volume (0.79–1.34 cm3 g−1) (Table 1). The nitrogen adsorption–desorption curves of the samples are typical of Type IV isotherms. Hysteresis loops occur at P/P° ∼0.6–0.8, indicative of mesopores between 4–8 nm (Fig. 2). The vertical and almost parallel adsorption and desorption branches for all samples except 10Zr-100-24 show that the pores are narrowly distributed with a spread of 0.5 to 1 nm about the mean pore size.
The pores in 10Zr-100-24, which was prepared with low water content in the synthesis gel, had a wider distribution, from 3.5 to 8.4 nm. The amount of water in the synthesis gel affects the pore size. As the water content in the synthesis gel increased, the resulting materials had bigger pores. The mean pore diameter in Zr-208-24 was 6.3 nm and increased to 8.0 nm for Zr-639-24.
From the isotherms of the calcined oxides, an aging time of 8 h is sufficient to form ordered mesopores (Fig. S2†). The samples show a Type IV isotherm with parallel hysteresis loops at P/P° of around 0.7–0.8, corresponding to a narrow pore size distribution centered at ∼7.0 nm. Increasing the aging time from 8 to 24 h led to a decrease in the surface area and total pore volume without affecting the pore size.
 | ||
| Fig. 2 (a) N2 adsorption–desorption isotherms and (b) pore size distribution curves of Zr-SBA-15 samples prepared with different water content. Isotherms are offset by 300 cm3 g−1. | ||
The effect of water on the interaction of the Pluronic template with silicate was probed by thermogravimetry. The as-synthesized zirconium-containing samples lost weight between ∼200 °C and 550 °C (Fig. 3). The total weight loss is in the order 30–50%. The evolution of CO2 and H2O during weight loss was verified by TGA-MS (Fig. S3†), hence this step is associated with the decomposition of Pluronic. In contrast, decomposition of the purely siliceous SBA-15 occurred at a lower temperature, ∼150–300 °C. This difference in the decomposition temperature for Pluronic suggests that the interaction of the template with the zirconium-containing silica surface is stronger than with pure silica. With an increased amount of water in the synthesis gel, the decomposition started at lower temperatures, suggesting that the interaction between the template and the zirconium species in the mesostructure is weakened. This agrees with the pore size expansion when the water content in the synthesis gel was increased. Chen et al.42 observed that samples with low zirconium content, Si/Zr > 20, decomposed in two temperature regimes, between 180–200 °C and at a higher temperature of 270–300 °C. However, samples containing more zirconium (Si/Zr of 16.7 and 10) showed only the higher temperature decomposition, indicating a stronger interaction between the surface and Pluronic.
 | ||
| Fig. 3 Weight loss and DTG profiles of as-synthesized samples of (a) Si-SBA-15 (b) 10Zr-100-24 (c) 10Zr-208-24 (d) 10Zr-423-24 and (e) 10Zr-639-24. | ||
The SEM images showed that the Zr-SBA-15 samples formed hexagonal platelets (Fig. 4). With a longer aging time of 24 h, the platelets were bigger in diameter (2–2.5 μm) than those prepared with 8 h aging, although the thickness was unaffected (∼ 340 nm) The formation of plate-shaped SBA-15 has been attributed to a quick condensation of silicate around the micelles which reduces the interconnection between the particles.42 The presence of Zr4+ is important as it accelerates the self-assembly of Pluronic micelles and TEOS. When the amount of water in the synthesis gel is decreased, the platelets appeared to agglomerate into bigger secondary particles. For 10ZrMPS, only big agglomerates of ∼10 μm were observed instead of platelets.
 | ||
| Fig. 4 SEM images of (a) 10Zr-100-24 (b) 10Zr-208-24 (c) 10Zr-423-24 (d) 10Zr-639-24 (e) 10Zr-423-8 and (f) 10ZrMPS. | ||
The extent of zirconium incorporation into the silica framework was found to depend on the water content of the gel and the aging time. When the amount of water in the synthesis gel was increased at constant HCl volume, more zirconium could be incorporated into the silica matrix (Table 2). This can be attributed to a decrease in the solubility of the zirconium species as the pH of the gel rose from 0.17 to 0.37. However, the zirconium content in the solids was still lower than expected, with Si/Zr between 13.1 to 18.4, instead of Si/Zr of 10. When the aging time was increased from 8 h to 24 h, the Si/Zr ratio rose from 12.9 to 17.9. The lower zirconium in the 24 h-aged samples showed that under the acidic conditions the rate of dissolution into the synthesis gel is higher than the rate of deposition.
| Sample | Si/Zr | pHa | Acidityb (mmol g−1) | Lewis/Brønstedc | |
|---|---|---|---|---|---|
| Solid | 25 °C | 100 °C | |||
| a In the synthesis gel. b From NH3 TPD. c From pyridine IR measurements. N.D: not determined. | |||||
| 10Zr-100-24 | 18.4 | 0.17 | 0.17 | 12.5 | 3.3 |
| 10Zr-208-24 | 17.9 | 0.33 | 0.31 | 11.2 | 4.3 |
| 10Zr-423-24 | 14.6 | 0.65 | 0.33 | 14.5 | 4.1 |
| 10Zr-639-24 | 13.1 | 0.73 | 0.43 | 15.7 | 6.1 |
| 10Zr-208-8 | 12.9 | 0.33 | 0.51 | N.D. | N.D. |
| 10Zr-423-8 | 16.4 | 0.65 | 0.35 | N.D. | N.D. |
| 10ZrMPS | 15.4 | — | 0.32 | N.D. | N.D. |
| Zr-beta | 100 | — | — | 8.3 | 2.5 |
The incorporation of zirconium into the silica framework is supported by FT-IR measurements. The FT-IR spectra of the samples showed a number of bands indicative of Si–O bonding (Fig. 5). In Si-SBA-15, an intense vibration band at ca. 980–1110 cm−1 with a shoulder at ca. 1220 cm−1 can be assigned to Si–O–Si asymmetric stretching modes while the smaller band at ca. 935 cm−1 is due to the Si–OH group vibration. These bands broadened and merged together in the zirconium-containing samples. The smaller bands centered at 800 and 458 cm−1 are assigned to Si–O–Si symmetric stretching and rocking modes, respectively, and they also broadened with a slight decrease in intensity when zirconium was introduced into the silica framework. The 29Si MAS spectra show peaks of Q4 and Q3 (Si with four and three neighbouring Si) with only a small Q2 peak. The higher intensity of the Q3 peak over Q2 supports the presence of isolated zirconium ions in the silica matrix (Fig. S4†).
 | ||
| Fig. 5 FTIR of (a) SBA-15 (b) 10Zr-100-24 (c) 10Zr-208-24 (d) 10Zr-423-24 (e) 10Zr-639-24 (f) 10Zr-208-8 and (g) 10Zr-423-8. | ||
The acidity of the samples was assessed by ammonia temperature-programmed desorption (Fig. 6). Desorption of ammonia occurred between 170 and 470 °C, showing that the samples have a range of acidic sites. The density of acid sites increased from 0.17 mmol g−1 in 10Zr-100-24 to 0.43 mmol g−1 for 10Zr-639-24. This is due to the incorporation of more zirconium into the silica framework as the water content in the synthesis gel increased. The pyridine IR measurements indicate the presence of both Brønsted and Lewis acid sites in the samples (Fig. S5†). The adsorption of pyridine at Lewis acid sites is indicated by bands at ∼1440–1450 cm−1 and 1600–1610 cm−1, while the pyridinium ion formed by adsorption of pyridine at Brønsted acid sites shows a band at ∼1540–1550 cm−1. The peak at ∼1491 cm−1 is attributed to both Brønsted and Lewis acidities. The incorporation of zirconium into the silica framework can result in the generation of Brønsted acid sites as suggested by Tanabe et al.45 The relative density of Lewis/Brønsted acid sites were obtained from the bands ∼1445 cm−1 and ∼1545 cm−1 after normalizing with the respective molar extinction coefficients.46 Lewis acidity was predominant in all the samples (Table 2). The density of Lewis acid sites increased with the amount of water in the synthesis gel while samples formed with less water had more Brønsted acidity. Evacuation of the samples at 100 °C resulted in the removal of pyridine bound to weak Lewis acid sites, so that the Lewis/Brønsted ratio decreased.
Catalytic activity
The catalytic activity of the 10Zr-SBA catalysts was tested in the liquid phase Prins condensation of paraformaldehyde and β-pinene to form Nopol (Table 3). The samples exhibited good activity. Conversions of 54–74% were obtained after 6 h when the reaction was carried out at 80 °C. At 100 °C, the conversion increased to 69–95%. The highest activity was observed for catalysts formed with H2O/Si of 208 and 423. For samples prepared at H2O/Si of 423, the conversion decreased from 74% in 10Zr-423-24 to 60% in 50Zr-423-24. Despite the different pore sizes of the platelet samples, Nopol was the only product formed. In contrast, the selectivity to Nopol over 10ZrMPS was only 67%, because isomerisation of the β-pinene led to by-products. Using microporous Zr-zeolite beta as the catalyst also resulted in a fast reaction rate and, after 2 h, the conversion was already at 77%. However, the selectivity to Nopol was only 46% as limonene and camphene were also formed. The reaction rate was even higher over the strongly acidic H-Beta (Si/Al 12.5) but the Nopol selectivity was only 4%.| Entry | Catalyst | Conv.a (%) | Nopol sel. (%) | Initial TOF (h−1) |
|---|---|---|---|---|
| a After 6 h. b After 2 h. Reaction conditions: 1 mmol β-pinene, 2 mmol paraformaldehyde, 5 ml toluene, 50 mg catalyst, 80 °C. | ||||
| 1 | 10Zr-100-24 | 63 | 100 | 23 |
| 2 | 10Zr-208-24 | 72 | 100 | 19 |
| 3 | 10Zr-423-24 | 74 | 100 | 16 |
| 4 | 10Zr-639-24 | 54 | 100 | 13 |
| 5 | 10Zr-208-8 | 74 | 100 | 18 |
| 6 | 10Zr-423-8 | 68 | 100 | 16 |
| 7 | 50Zr-423-24 | 64 | 100 | 42 |
| 8 | 10ZrMPS | 95 | 67 | 37 |
| 9 | Zr-betab | 77 | 46 | 163 |
| 10 | H-betab | 100 | 4 | 29 |
To optimize the reaction, the reaction temperature was varied using 10Zr-208-8 as the catalyst (Fig. 7). After 8 h, the conversion at 60 °C was 64% as compared to 77% when the reaction was carried out at 80 °C. Surprisingly, when the reaction temperature was increased to 100 °C, the initial rate was smaller than that at 80 °C, although conversion continued to increase with time to reach 95% after 8 h. The selectivity was unaffected by the higher temperature. The lower final conversion at temperatures below 80 °C indicates that the active sites are blocked, most probably by strong adsorption of the products. A higher temperature of 100 °C can help to remove the products that block the active sites, allowing the reaction to proceed.
 | ||
| Fig. 7 Conversion of β-pinene over 10Zr-208-8 at (▲) 60 (■) 80 (○) 100 and (□) 110 °C. | ||
However, due to the higher temperature, fewer reactants can adsorb at the surface of the catalyst, leading to a lower initial reaction rate. At an even higher reaction temperature of 110 °C, the conversion reached 95% in 4 h but mostly isomerisation products of β-pinene were formed, so that Nopol selectivity was only 8%. Under these conditions, the surface concentration of paraformaldehyde is low so that formation of camphene, limonene and other mono-isomers of β-pinene became predominant. This is supported by thermogravimetric measurements on 10Zr-423-8, where the differential thermogravimetric profile shows a high temperature peak between 160 and 270 °C for the desorption of β-pinene, in addition to desorption of weakly bound β-pinene below 150 °C (Fig. S6†). In contrast, the desorption of formaldehyde occurred below 150 °C. Indeed, the reaction order in paraformaldehyde was found to be 1.72 compared to 0.79 for β-pinene (Fig. S7†). The higher rate dependence on paraformaldehyde indicates that its adsorption at the surface of the catalyst is weaker than β-pinene.
The high selectivity to Nopol over the Zr-SBA-15 platelets may in part be attributed to a unique combination of acid sites with a predominance of Lewis over Brønsted acid sites (Lewis/Brønsted ∼ 3.3–6.1). Zeolite H-Beta, which has strong Brønsted acidity, gives a low selectivity to Nopol. Similarly, our previous investigations into the catalytic properties of HITQ-2, a MWW zeolite, had found that as a consequence of its higher density of Brønsted acid sites (Lewis/Brønsted 1.3), no Nopol was formed.43 Instead, the products were all mono-isomers of β-pinene. These results agree well with that for the intramolecular Prins reaction where citronellal is cyclised to isopulegols.24 There, we showed that catalysts that combined strong Lewis with weak Brønsted acid sites had good activity and selectivity to isopulegols. Strongly acidic catalysts such as Amberlyst, Nafion and sulfated zirconia also gave high conversions but the products were mainly due to dehydration, cracking and etherification whereas silica, a weak acid, gave only very low conversion.24 The coordinatively unsaturated zirconium ion in Zr-SBA-15 is a much stronger Lewis acid site compared to silicon. Coordination of β-pinene and formaldehyde to the zirconium brings the molecules close together (Scheme 2). The platelet morphology exposes high numbers of coordinatively unsaturated zirconium ions at edge sites, whereas in a granular sample most of the active sites are located deep within the channels. The edge sites allow more flexibility for the substrate molecules to orient themselves for intermolecular reaction. Hence, it is envisaged that steric hindrance should not be a major factor (as evidenced from the rate studies) despite the bulky size of β-pinene (Fig. S8†). After the molecules are adsorbed and coordinated to the Zr center, the reaction is initiated when hydrogen is removed by a neighbouring oxygen. This is followed by the formation of the C–C bond. The binding of the two reactants has to be balanced because too strong a binding of either one will adversely affect the rate and selectivity of the reaction. The lower reaction order in β-pinene indicates that it adsorbs stronger than formaldehyde at the surface of the catalyst. In the presence of Brønsted acid sites, isomerisation leads to side products such as camphene and limonene. However, the strength and density of acidic sites for 10ZrMPS is very similar to that of the 10Zr-SBA-15 platelets and therefore unlikely to be solely responsible for the low Nopol selectivity. Moreover, the pores of 10ZrMPS are in the order of ∼3–8 nm, and, although not narrowly distributed, they are within the pore size range of the Zr-SBA-15 platelets. Hence, a plausible explanation for the lower Nopol selectivity over 10ZrMPS may lie in its big particle sizes, where in diffusing through the long tortuous channels, β-pinene encounters several acidic sites for isomerisation. Similarly, the higher density of Brønsted acid sites in Zr-beta and H-beta compared to the 10Zr-SBA-15 samples and the diffusion limitation posed by the microporous nature of zeolites could contribute to the lower Nopol selectivity. The short mesoporous channels in Zr-SBA-15 platelets offer easy access to the active sites leading to improved activity and utilization of the catalyst.
 | ||
| Scheme 2 Proposed mechanism for the intermolecular Prins reaction and isomerisation of β-pinene. | ||
Reuse of the catalyst
The recovery and reusability of the catalysts were investigated. After the reaction, the catalyst was washed with toluene, centrifuged and dried in the oven for the next run. The results showed that the activity was lower. It would appear that some active sites remained blocked by the residual products. Treating the used catalyst with H2O2 solution at 40 °C overnight resulted in a rejuvenated catalyst with activity > 95% conversion and 100% selectivity to Nopol (Fig. 8). With H2O2, fouling compounds that cannot be removed by washing with toluene can be oxidized at low temperatures. The differential thermogravimetric profile of the used catalyst confirmed that, despite washing with toluene, small amounts of strongly adsorbed residues were still present and could only be removed at temperatures of up to 500 °C (Fig. S9†). | ||
| Fig. 8 Reuse of catalyst 10Zr-208-8 (■) conversion after 8 h (□) selectivity. | ||
Conclusions
The amount of water present during the synthesis of Zr-SBA-15 affects the dimension and distribution of pores. Samples prepared with a H2O/Si ratio of 208–639 formed platelets with pores that are narrowly distributed about a mean pore size. In contrast, the sample prepared using a H2O/Si ratio of 100 had a broader pore size distribution. The mean pore diameter varied from 4 to 8 nm, depending on the amount of water in the synthesis gel. The expansion in pore size with increased water in the synthesis gel was attributed to a reduced interaction of the Pluronic template with the inorganic surface. The Si/Zr in the calcined oxides decreased from 18.4 to 13.1 when the H2O/Si ratio was increased from 100 to 639. The increased zirconium content in the samples resulted in a higher density of Brønsted and Lewis acid sites, with a predominance of the latter. The Zr-SBA-15 platelets showed good catalytic activity in the Prins condensation of β-pinene and paraformaldehyde. The selectivity to Nopol was essentially 100%. In comparison, the selectivity over particulate 10ZrMPS, microporous Zr-beta and H-beta was lower. The catalysts could be regenerated for further batch reactions following treatment with H2O2.Acknowledgements
Financial support from National University of Singapore under grant number R-143-000-418-112 is gratefully acknowledged.Notes and references
- K. Wilson and J. H. Clark, Pure Appl. Chem., 2000, 72, 1313–1319 CrossRef CAS
.
-
B. B. Snider, in Comprehensive Organic Synthesis, ed. B. M. Trost, I. Fleming and C. H. Heathcock, Pergamon, Oxford, 1991, vol. 2, pp. 527–561 Search PubMed
.
- M. L. Clarke and M. B. France, Tetrahedron, 2008, 64, 9003–9031 CrossRef CAS
.
- I. M. Pastor and M. Yus, Curr. Org. Chem., 2007, 11, 925–957 CrossRef CAS
.
- J. T. Williams, P. S. Bahia and J. S. Snaith, Org. Lett., 2002, 4, 3727–3730 CrossRef CAS
.
- B. B. Snider and G. B. Phillips, J. Org. Chem., 1983, 48, 464–469 CrossRef CAS
.
- L. M. Stephenson and M. Orfanopoulos, J. Org. Chem., 1981, 46, 2200–2201 CrossRef CAS
.
- H. Kwart and M. Brechbiel, J. Org. Chem., 1982, 47, 5409–5411 CrossRef CAS
.
- N. H. Andersen, S. W. Hadley, J. D. Kelly and E. R. Bacon, J. Org. Chem., 1985, 50, 4144–4151 CrossRef CAS
.
- J. S. Yadav, B. V. S. Reddy and G. Bhaishya, Green Chem., 2003, 5, 264–266 RSC
.
- G. X. Li, Y. L. Gu, Y. Ding, H. P. Zhang, J. M. Wang, Q. Gao, L. Yan and J. S. Suo, J. Mol. Catal. A: Chem., 2004, 218, 147–152 CrossRef CAS
.
-
H. Surburg and J. Panten, Common Fragrance and Flavor Materials, Preparation, Properties and Uses, Wiley-VCH, Weinheim, Germany, 2006, pp. 67–68 Search PubMed
.
- J. P. Bain, J. Am. Chem. Soc., 1946, 68, 638–641 CrossRef CAS
.
- A. L. Villa de P, E. Alarcón and C. M. de Correa, Chem. Commun., 2002, 2654–2655 RSC
.
- A. L. Villa de P, E. Alarcón and C. M. de C, Catal. Today, 2005, 107–108, 942–948 CrossRef CAS
.
- M. Selvaraj and P. K. Sinha, New J. Chem., 2010, 34, 1921–1929 RSC
.
- E. A. Alarcón, L. Correa, C. Montes and A. L. Villa, Microporous Mesoporous Mater., 2010, 136, 59–67 CrossRef
.
- M. Selvaraj and Y. Choe, Appl. Catal., A, 2010, 373, 186–191 CrossRef CAS
.
- A. Corma and M. Renz, Arkivoc, 2007, viii, 40–48 Search PubMed
.
- U. R. Pillai and E. Sahle-Demessie, Chem. Commun., 2004, 826–827 RSC
.
- A. M. Selvaraj and S. Kawi, J. Mol. Catal. A: Chem., 2006, 246, 218–222 CrossRef
.
- M. K. Yadav and R. V. Jasra, Catal. Commun., 2006, 7, 889–895 CrossRef CAS
.
- M. V. Patil, M. K. Yadav and R. V. Jasra, J. Mol. Catal. A: Chem., 2007, 273, 39–47 CrossRef CAS
.
- G. K. Chuah, S. H. Liu, S. Jaenicke and L. J. Harrison, J. Catal., 2001, 200, 352–359 CrossRef CAS
.
- Y. Z. Zhu, Y. T. Nie, S. Jaenicke and G. K. Chuah, J. Catal., 2005, 229, 404–413 CrossRef CAS
.
- S. Telalović, J. F. Ng, R. Maheswari, A. Ramanathan, G. K. Chuah and U. Hanefeld, Chem. Commun., 2008, 4631–4633 RSC
.
- S. Telalović, A. Ramanathan, J. F. Ng, R. Maheswari, C. Kwakernaak, F. Soulimani, H. C. Brouwer, G. K. Chuah, B. M. Weckhuysen and U. Hanefeld, Chem.–Eur. J., 2011, 17, 2077–2088 CrossRef
.
- M. Morandin, R. Gavagnin, F. Pinna and G. Strukul, J. Catal., 2002, 212, 193–200 CrossRef CAS
.
- M. S. Wong, H. C. Huang and J. Y. Ying, Chem. Mater., 2002, 14, 1961–1973 CrossRef CAS
.
- Y. Z. Zhu, S. Jaenicke and G. K. Chuah, J. Catal., 2003, 218, 396–404 CrossRef CAS
.
- S. Y. Chen, J. F. Lee and S. Cheng, J. Catal., 2010, 270, 196–205 CrossRef CAS
.
- A. Ramanathan, M. C. C. Villalobos, C. Kwakernaak, S. Telalović and U. Hanefeld, Chem.–Eur. J., 2008, 14, 961–972 CrossRef CAS
.
- D. Barreca, M. P. Copley, A. E. Graham, J. D. Holmes, M. A. Morris, R. Seraglia, T. R. Spalding and E. Tondello, Appl. Catal., A, 2006, 304, 14–20 CrossRef CAS
.
- O. Y. Gutierrez, G. A. Fuentes, C. Salcedo and T. Klimova, Catal. Today, 2006, 116, 485–497 CrossRef CAS
.
- W. M. Hua, Y. H. Yue and Z. Gao, J. Mol. Catal. A: Chem., 2001, 170, 195–202 CrossRef CAS
.
- H. Matsuhashi, M. Tanaka, H. Nakamura and K. Arata, Appl. Catal., A, 2001, 208, 1–5 CrossRef CAS
.
- C. L. Chen, S. F. Cheng, H. P. Lin, S. T. Wong and C. Y. Mou, Appl. Catal., A, 2001, 215, 21–30 CrossRef CAS
.
- B. L. Newalkar, J. Olanrewaju and S. Komarneni, J. Phys. Chem. B, 2001, 105, 8356–8360 CrossRef CAS
.
- K. Szczodrowski, B. Prelot, S. Lantenois, J. Zajac, M. Lindheimer, D. Jones, A. Julbe and A. van der Lee, Microporous Mesoporous Mater., 2008, 110, 111–118 CrossRef CAS
.
- Y. C. Du, S. Liu, Y. L. Zhang, F. Nawaz, Y. Y. Ji and F. S. Xiao, Microporous Mesoporous Mater., 2009, 121, 185–193 CrossRef CAS
.
- S. Y. Chen, L. Y. Jang and S. Cheng, Chem. Mater., 2004, 16, 4174–4180 CrossRef CAS
.
- S. Y. Chen, C. Y. Tang, W. T. Chuang, J. J. Lee, Y. L. Tsai, J. C. C. Chan, C. Y. Lin, Y. C. Liu and S. Cheng, Chem. Mater., 2008, 20, 3906–3916 CrossRef CAS
.
- J. Wang, S. Jaenicke, G. K. Chuah, W. M. Hua, Y. H. Yue and Z. Gao, Catal. Commun., 2011, 12, 1131–1135 CrossRef CAS
.
- A. Vinu, V. Murugesan, W. Bolhlmann and M. Hartmann, J. Phys. Chem. B, 2004, 108, 11496–11505 CrossRef CAS
.
-
K. Tanabe, M. Misono, Y. Ono and H. Hattori, New Solid Acids and Bases, Their Catalytic Properties, in Studies in Surface Science and Catalysis 51, ed. B. Delmon and J. T. Yates, Kodansha, Tokyo, 1989 Search PubMed
.
- C. A. Emeis, J. Catal., 1993, 141, 347–354 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S6. See DOI: 10.1039/c2cy20084h |
| This journal is © The Royal Society of Chemistry 2012 |

