Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance†
Fan
Dong
*a,
Yanjuan
Sun
a,
Liwen
Wu
a,
Min
Fu
a and
Zhongbiao
Wu
*b
aCollege of Environmental and Biological Engineering, Key Laboratory of Catalysis Science and Technology of Chongqing Education Commission, Chongqing Technology and Business University, Chongqing, 400067, China. E-mail: dfctbu@126.com
bDepartment of Environmental Engineering, Zhejiang University, Hangzhou, 310027, China. E-mail: zbwu@zju.edu.cn
First published on 20th April 2012
Abstract
Polymeric nitrogen-rich graphitic carbon nitride (g-C3N4-T) nanocatalyst was synthesized for the first time with a facile approach by directly treating low cost thiourea in air. The g-C3N4-T demonstrated much higher visible light photocatalytic activity than that of g-C3N4 obtained from toxic dicyandiamide, C-doped TiO2 and P25.
Challenges of environmental pollution and energy shortage faced by human beings have raised awareness of a potential global crisis. Among the various technologies to tackle the challenges, semiconductor photocatalysis has emerged as one of the most promising technologies because it can effectively utilize the abundant energy of either natural sunlight or artificial indoor illumination.1 The mostly applied traditional TiO2 photocatalyst can only utilize UV light (4–5% of the total solar energy) due to its wide band gap (3.0–3.2 eV).2 In recent years, intensive efforts have been devoted to modifying TiO2, including nonmetal doping, hetero-junction construction and quantum dots or dye sensitization in order to make it visible light active.3 Some complex metal oxides as new visible light photocatalytic materials including tungstates, niobates, tantalates, vandates and carbonates have been successfully developed recently.4 A number of sulfides, nitrides and oxynitrides have also been synthesized as alternative materials for efficient visible light photocatalysis.5
Very recently, novel metal-free polymeric graphitic carbon nitride (g-C3N4) prepared from cyanamide was identified as a high performance photocatalyst for the production of hydrogen from water and degradation of organic pollutants under visible light irradiation.6 The g-C3N4 with a narrow band gap (2.7 eV) could utilize visible light directly without modification. The search for suitable precursors for better synthesis of g-C3N4 is a key issue. In general, the commonly used precursors are nitrogen-rich compounds containing pre-bonded triple or double C–N core structure, such as cyanamide, dicyandiamide, trithiocyanuric acid, melamine, triazine and heptazine derivatives.7 Some of the precursor compounds are difficult to obtain and expensive, and some of them are unstable and even highly explosive. Zou et al. transformed urea into g-C3N4 over mesoporous TiO2 spheres followed by HF etching.8 The precursor urea, a nitrogen-rich compound, is cheap and easily available.8 However, such a process to prepare g-C3N4 with the assistance of pre-synthesized TiO2 is complicated and use of HF is not environmentally benign.
Thus, it is highly desirable to develop a facile and environmental benign approach to synthesize g-C3N4 from cheap and nontoxic precursors for large scale applications.3b We have recently developed an efficient synthesis method for g-C3N4 by directly heating urea without employing additional assistance.9 There is, however, still a challenge to explore other suitable green precursors to synthesize g-C3N4. The present work reports the first facile synthesis of polymeric nitrogen-rich g-C3N4 nanostructure by direct treating thiourea at 550 °C for 2 h, where thiourea is a common and low cost raw material in chemical industry substituting the widely used toxic or unstable precursors. The as-prepared ordered g-C3N4 from thiourea exhibited high visible light photocatalytic activity for degradation of organic pollutant compared with g-C3N4 prepared from toxic dicyandiamide. The high activity can be ascribed to the increased visible light absorbance and promoted charge separation. As this method is facile, economic and environmental benign, it is highly attractive and ready for large scale environmental and energetic applications.
The XRD patterns of the samples prepared from thiourea and dicyandiamide (Fig. 1a) can be indexed to g-C3N4. The strong interplanar stacking peak of aromatic systems at around 27.5° is indexed to (002) peak.6a The peak at 13.0° corresponding to in-plane ordering of tri-s-triazine units with a period of 0.675 nm which form 1D melon strands.9Fig. 1b further implies that the characteristic (002) peak shifts from 27.50 for g-C3N4-T to 27.75 for g-C3N4-D, indicating the average interlayer distance of g-C3N4-T (d = 0.326 nm) is slightly larger than that of g-C3N4-D (d = 0.321 nm). The (002) peak of g-C3N4-T is wider and lower than g-C3N4-D. These results suggests that g-C3N4-T sample has less degree of condensation and smaller domain size.6b
 | ||
| Fig. 1 XRD pattern of g-C3N4-T and g-C3N4-D (a) and enlarged view of (002) peak (b). | ||
Wang et al. prepared g-C3N4 photocatalyst from cyanamide.6a The triple C–N bond in cyanamide played a key role in the formation of g-C3N4.6,7 In the present work, the chemical structure of thiourea doesn’t contain a triple C–N bond, which implies that the formation mechanism of g-C3N4 from thiourea is totally different from that of cyanamide. However, the fundamental chemical reactions involving the formation mechanism of g-C3N4 from thiourea is rather complicated. A systematic investigation is currently underway to elucidate the chemical mechanism and it will be reported in future work.
The detailed discussion on XPS can be found in ESI (Fig. S1†). Fig. S1d† shows that the atomic ratio of hydroxyl groups to the total three kinds of oxygen contributions is 42.4% for g-C3N4-T, much higher than that of g-C3N4-D (23.3%). The increase in surface hydroxyl content is advantageous for trapping more photo-generated holes and thus preventing electron hole recombination.10 The atomic ratios of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O of g-C3N4-T and g-C3N4-D was determined to be 1
O of g-C3N4-T and g-C3N4-D was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.40
1.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.021 and 1
0.021 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.37
1.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.021 from XPS, respectively. The nitrogen content is higher than the stoichiometry in C3N4, suggesting the g-C3N4-T and g-C3N4-D samples are rich in nitrogen. The excess nitrogen can be attributed to uncondensed amino groups during the condensation of thiourea.7h
0.021 from XPS, respectively. The nitrogen content is higher than the stoichiometry in C3N4, suggesting the g-C3N4-T and g-C3N4-D samples are rich in nitrogen. The excess nitrogen can be attributed to uncondensed amino groups during the condensation of thiourea.7h
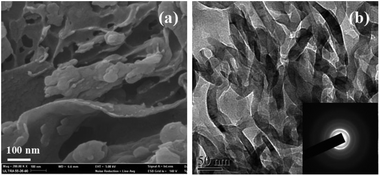
The SEM image of g-C3N4-D in Fig. S2a† shows that some irregular stacks consisting of layers and particles are formed. The high magnification in Fig. S2b† indicates that some nanoparticles are attached on the layers with a thickness of 10 nm. For the g-C3N4-T sample, large amounts of layers with different sizes are stacked together (Fig. S3†). The curved layers are smooth and interconnected with pores of different sizes (Fig. 2a). The thickness of the layers is about 15 nm. The structural morphology was further investigated by TEM. Fig. S4a and S4b† show that g-C3N4-D are composed of numerous randomly organized nanosheets and nanoparticles with irregular shape. The nanosheets are very thin and transparent to the electron beam. The inset in Fig. S4a† shows the corresponding SAED pattern with clear diffraction rings. Observing carefully at the TEM images for g-C3N4-T (Fig. 2b), one can see the smooth and flat layers with ordered structure. The ordered non-crystalline structure goes consistently with the XRD pattern. The SAED in Fig. 2b indicates that diffraction rings of g-C3N4-T are not as distinct as that of g-C3N4-D, consistent with the widened (002) peak in Fig. 1b.
Fig. S5a† shows that the isotherms of g-C3N4-D are close to type IV and the isotherms of g-C3N4-T can be classified as type II. The corresponding pore-size distribution curves (Fig. S5b†) indicate the presence of mesopores for the two g-C3N4 samples, which can be ascribed to the aggregation of nanosheets or particles. The mesoporous structure is proved to facilitate the transportation of reactants and products and to favor the harvesting of photo-energy (See detailed discussion following Fig. S5†).11
The UV-vis DRS of g-C3N4 obtained from thiourea and dicyandiamide are shown in Fig. 3a. The g-C3N4-T exhibits stronger visible light absorption in the range of 450 to 700 nm than that of g-C3N4-D. The band gap energy (Eg) estimated from the intercept of the tangents to the plots of (αhν)1/2vs. photon energy are 2.46 and 2.58 eV for g-C3N4-T and g-C3N4-D, respectively (Fig. 3b), which are smaller than the literature value (2.7 eV). The enhanced visible light absorbance and reduced band gap of the g-C3N4-T obtained from thiourea can be attributed to the enriched nitrogen content, which modified the band structure. Generally, the photocatalytic activity of a semiconductor is proportional to (IαΦ)n, where Iα is the photo numbers absorbed by photocatalyst per second and Φ is the efficiency of the band gap transition.9 The increased visible light absorbance could contribute to the increase of IαΦ, thus enhancing the photocatalytic activity.
Fig. S6† shows the room temperature PL spectra of g-C3N4-T and g-C3N4-D. The PL intensity of g-C3N4-D is much higher than that of g-C3N4-T, implying that the separation of photogenerated electrons and holes is enhanced on g-C3N4-T. It was found that 2D nanostructures favored the transfer of electrons and holes on the crystal surface and promoted the charge separation.12 Thus, the 2D layered nanostructure (Fig. 2a) and increased surface hydroxyl content (Fig. S1d†) of the g-C3N4-T facilitate the separation of charge carriers, thus contributing to the lower PL intensity.
The precursors employed to synthesize g-C3N4 semiconductor photocatalyst usually contain triple or double C–N bonds. Wang et al. prepared g-C3N4 by condensation of trithiocyanuric acid which exhibited a moderate rate of photocatalytic H2 evolution.7c Li et al. fabricated g-C3N4 by directly heating helamine and the as-prepared g-C3N4 showed good performance in photodegradation of organic pollutant.7d Domen synthesized a carbon nitride structure for visible-light catalysis by copolymerization of dicyandiamide and barbituric acid.7e The results indicate that g-C3N4 is an excellent visible light driven photocatalyst.
Fig. 4a shows adsorption property and visible light photocatalytic performance of g-C3N4-T, g-C3N4-D, C-doped TiO2 and P25 for degradation of contaminant RhB. Visible photocatalytic degradation of organic dyes was well documented. The adsorption–desorption equilibrium was reached within 60 min. The amounts of RhB adsorbed by the g-C3N4-T with low SBET is highest, indicating that g-C3N4-T has a strong power for RhB adsorption. When there is no photocatalyst involved with visible light irradiation, the RhB is stable, indicating that self-degradation was negligible. The RhB undergoes pronounced obvious decomposition in the presence of photocatalysts under visible light irradiation. P25 exhibits decent visible light activity with a removal ratio of 18.6% due to the existence of the rutile phase. The band gap of rutile TiO2 is 3.0 eV and can be excited by visible light with wavelengths shorter than 413 nm. Although the C-doped TiO2 has a higher SBET and a larger pore volume than that of g-C3N4-T and g-C3N4-D, the visible light activities of g-C3N4 samples are even higher than that of the high-performance C-doped TiO2 (Table S1†), which implies that g-C3N4 is an efficient photocatalyst for degradation of organic pollutants.6a,9 Furthermore, the g-C3N4-T prepared from thiourea exhibits much more efficient photocatalytic activity (removal ratio of 70.9% and initial rate constant of 0.0031 min−1) than that of g-C3N4 prepared from dicyandiamide (removal ratio of 49.3% and rate constant of 0.0018 min−1). The enhanced visible light absorbance (Fig. 3a) and promoted charge separation (Fig. S6†) of g-C3N4-T directly contributed to the outstanding visible light photocatalytic activity.10,11 These facts also suggest that thiourea is a better precursor than toxic dicyandiamide.
 | ||
| Fig. 4 The adsorption property and visible light photocatalytic performance of g-C3N4-T, g-C3N4-D and C-doped TiO2 (a) and repeated photocatalytic activities (b) for degradation of RhB. | ||
To test the stability of g-C3N4-T on photocatalytic RhB degradation, multiple runs of photocatalysis are carried out (Fig. 4b). It is interesting to find that the novel g-C3N4-T could maintain efficient and durable visible light photocatalytic activities after five cycles of repeated runs with no obvious deactivation. The results suggest that g-C3N4-T photocatalyst is both stable and efficient, which is important for practical applications. For the first time, thiourea was facilely transformed to g-C3N4 polymer materials with high visible light activity and stability. The as-prepared g-C3N4 materials can also be applied in solar energy conversion and fine chemicals synthesis.6a,7e As the low-cost thiourea precursor is easily available and the synthesis method is simple, it is extremely attractive and valuable for large scale environmental and energetic applications.
Polymeric nitrogen-rich g-C3N4 layered nanostructure was synthesized with a facile and environmental benign approach by directly treating low-cost thiourea in air at 550 °C. Thiourea is a better precursor for the synthesis of g-C3N4 polymer than toxic dicyandiamide. The curved thin smooth layers of g-C3N4-T were interconnected, leading to the formation of mesoporous structure. The as-prepared polymeric g-C3N4 from thiourea exhibited high photocatalytic activity and stability under visible light irradiation, exceeding that of g-C3N4-D, C-doped TiO2 and P25. The enhanced visible light absorbance due to enriched nitrogen and promoted charge separation due to the 2D layered nanostructure and abundant surface hydroxyl groups are responsible for the outstanding photocatalytic performance of g-C3N4-T. This work demonstrates a highly valuable facile method to synthesize high-performance g-C3N4 polymeric photocatalysts from easy-available thiourea for large scale environmental and energetic applications.
Acknowledgements
This research is supported by the NSFC (51108487), “863 Program” of China (2010AA064905), the Program for Talented Young Teachers (Chongqing, 2011), Chongqing Education Commission (KJTD201020, KJZH11214) and the key discipline development project of CTBU (1252001).Notes and references
- (a) Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC; (b) C. Chen, W. H. Ma and J. C. Zhao, Chem. Soc. Rev., 2010, 39, 4206 RSC; (c) G. Liu, J. C. Yu, G. Q. Lu and H. M. Cheng, Chem. Commun., 2011, 47, 6763 RSC; (d) Q. Zhang, G. S. Li and J. C. Yu, J. Mater. Chem., 2010, 20, 4529 RSC; (e) B. F. Machado and P. Serp, Catal. Sci. Technol., 2012, 2, 54 RSC; (f) Q. j. Xiang, J. G. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782 RSC; (g) S. W. Liu, J. G. Yu and M. Jaroniec, Chem. Mater., 2011, 23, 4085 CAS.
- (a) Fujishima, X. T. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS; (b) G. Liu, L. Z. Wang, H. G. Yang, H. M. Cheng and G. Q. Lu, J. Mater. Chem., 2010, 20, 831 RSC.
- (a) R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS; (b) F. Dong, S. Guo, H. Q. Wang, X. F. Li and Z. B. Wu, J. Phys. Chem. C, 2011, 115, 13285 CrossRef CAS; (c) Z. Xu, I. Tabata, K. Hirogaki, K. Hisada, T. Wang, S. Wang and T. Hori, Catal. Sci. Technol., 2011, 1, 397 RSC; (d) F. Dong, H. Q. Wang, S. Guo, Z. B. Wu and S. C. Lee, J. Hazard. Mater., 2011, 187, 509 CrossRef CAS; (e) R. S. Dibbell, D. G. Youker and D. F. Watson, J. Phys. Chem. C, 2009, 113, 18643 CrossRef CAS.
- (a) X. Xiao and W. D. Zhang, J. Mater. Chem., 2010, 20, 5866 RSC; (b) S. S. Dunkle and K. S. Suslick, J. Phys. Chem. C, 2009, 113, 10341 CrossRef CAS; (c) Z. G. Zou, J. H. Ye, K. Sayama and H. Arakawa, Nature, 2001, 414, 625 CrossRef CAS; (d) J. Q. Yu and A. Kudo, Adv. Funct. Mater., 2006, 16, 2163 CrossRef CAS; (e) F. Dong, W. K. Ho, S. C. Lee, Z. B. Wu, M. Fu, S. C. Zou and Y. Huang, J. Mater. Chem., 2011, 21, 12428 RSC.
- (a) S. X. Ge and L. Z. Zhang, Environ. Sci. Technol., 2011, 45, 3027 CrossRef CAS; (b) J. R. Ran, J. G. Yu and M. Jaroniec, Green Chem., 2011, 13, 2708 RSC; (c) K. Maeda and K. Domen, Chem. Mater., 2010, 22, 612 CrossRef CAS.
- (a) X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2008, 8, 76 CrossRef; (b) X. H. Li, J. S. Zhang, X. F. Chen, A. Fischer, A. Thomas, M. Antonietti and X. C. Wang, Chem. Mater., 2011, 23, 4344 CrossRef CAS.
- (a) B. Jürgens, E. Irran, J. Senker, P. Kroll, H. Müller and W. Schnick, J. Am. Chem. Soc., 2003, 125, 10288 CrossRef; (b) J. R. Holst and E. G. Gillan, J. Am. Chem. Soc., 2008, 130, 7373 CrossRef CAS; (c) J. Zhang, J. Sun, K. Maeda, K. Domen, P. Liu, M. Antonietti, X. Z. Fu and X. C. Wang, Energy Environ. Sci., 2011, 4, 675 RSC; (d) S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2009, 25, 10397 CrossRef CAS; (e) J. S. Zhang, X. F. Chen, K. Takanabe, K. Maeda, K. Domen, J. D. Epping, X. Z. Fu, M. Antonietti and X. C. Wang, Angew. Chem., Int. Ed., 2010, 49, 441 CrossRef CAS; (f) M. J. Bojdys, J. Mller, M. Antonietti and A. Thomas, Chem.–Eur. J., 2008, 14, 8177 CrossRef CAS; (g) Q. J. Xiang, J. G. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355 CrossRef CAS; (h) A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J. O. Muller, R. Schlogl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893 RSC.
- X. X. Zou, G. D. Li, Y. N. Wang, J. Zhao, C. Yan, M. Y. Guo, L. Li and J. S. Chen, Chem. Commun., 2011, 47, 1066 RSC.
- F. Dong, L. W. Wu, Y. J. Sun, M. Fu, Z. B. Wu and S. C. Lee, J. Mater. Chem., 2011, 21, 15171 RSC.
- (a) J. G. Yu, G. H. Wang, B. Cheng and M. H. Zhou, Appl. Catal., B, 2007, 69, 171 CrossRef CAS; (b) Y. Huang, W. K. Ho, S. C. Lee, L. Z. Zhang, G. S. Li and J. C. Yu, Langmuir, 2008, 24, 3510 CrossRef CAS; (c) J. G. Yu, H. G. Yu, B. Cheng, M. H. Zhou and X. J. Zhao, J. Mol. Catal. A: Chem., 2006, 253, 112 CrossRef CAS.
- (a) H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406 CrossRef CAS; (b) J. G. Yu, Y. R. Su and B. Cheng, Adv. Funct. Mater., 2007, 17, 1984 CrossRef CAS; (c) G. S. Li, D. Q. Zhang, J. C. Yu and M. K. H. Leung, Environ. Sci. Technol., 2010, 44, 4276 CrossRef CAS.
- (a) Z. H. Ai, W. K. Ho, S. C. Lee and L. Z. Zhang, Environ. Sci. Technol., 2009, 43, 4143 CrossRef CAS; (b) Zhang and Y. F. Zhu, Chem. Mater., 2005, 17, 3537 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Fig. S1–S6. Further discussions on XPS results in Fig. S1 and pore structure in Fig. S4. See DOI: 10.1039/c2cy20049j |
| This journal is © The Royal Society of Chemistry 2012 |