Submicrometre-scale polyaniline colloidal spheres: photopolymerization preparation using fluorescent carbon nitride dots as a photocatalyst†
Xiaoyun
Qin
a,
Sen
Liu
a,
Wenbo
Lu
a,
Haiyan
Li
a,
Guohui
Chang
a,
Yingwei
Zhang
a,
Jingqi
Tian
ab,
Yonglan
Luo
a,
Abdullah M.
Asiri
cd,
Abdulrahman O.
Al-Youbi
cd and
Xuping
Sun
*acd
aState Key Lab of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin, China. E-mail: sunxp@ciac.jl.cn; Fax: +86-431-85262065; Tel: +86-431-85262065
bGraduate School of the Chinese Academy of Sciences, Beijing 100039, China
cChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
dCenter of Excellence for Advanced Materials Research, King Abdulaziz University, Jeddah 21589, Saudi Arabia
First published on 22nd December 2011
Abstract
The present communication reports on a novel synthesis of submicrometre-scale polyaniline colloidal spheres (PANICSs) using fluorescent carbon nitride dots (CNDs) as a photocatalyst for the first time. The subsequent treatment of such colloidal spheres with an aqueous AgNO3 solution produces Ag nanoparticles (AgNPs) decorated PANICSs (AgNPs–PANICSs) composites. Such composites exhibit remarkable catalytic performance toward catalytic reduction of 4-nitrophenol and electrochemical reduction of H2O2.
In recent years, electrically conducting and intrinsically colored polymers are widely used in diverse applications.1 Polyaniline (PANI) is one of the most studied conducting polymers due to its chemical stability and relatively high conductivity.2 As a result, intensive effort has been put into synthesizing PANI with various dimensions, morphologies, and architectures to meet the demand for the design and construction of devices.3 Wan and co-workers have reported the synthesis of PANI one-dimensional microstructures using β-naphthalene sulfonic acid and ammonium persulfate as dopant and oxidant, respectively.4 Carswell et al. have fabricated PANI nanowires on a flat surface under the control of the adsorbed surfactants.5 Li et al. have prepared void PANI colloidal spheres (PANICSs) in the presence of a small amount of an initiator under hydrothermal conditions.6 So far, a variety of PANI micro/nano structures including tubes, fibers, wires and hollow spheres have been successfully synthesized through hydrothermal synthesis, template methods, electropolymerization, photopolymerization and so on.7 However, to the best of our knowledge, the synthesis of PANICSs via photopolymerization has not been addressed before.
On the other hand, carbon nitride materials have been the focus of materials research because of their unique properties and wide applications in catalysis, sensors, corrosion protection etc.8 More recently, we have successfully prepared fluorescent carbon nitride dots (CNDs) by polymerization of CCl4 and 1,2-ethylenediamine under reflux, microwave, or solvothermal heating.9 In this communication, we demonstrate the first preparation of submicrometre-scale PANICSs by photopolymerization of aniline monomers using CNDs as an effective photocatalyst. The PANICSs were subsequently decorated with Ag nanoparticles (AgNPs) by in situ chemical reduction of Ag+ ions, leading to AgNPs–PANICSs composites. Such composites were found to exhibit remarkable catalytic performance toward catalytic reduction of 4-nitrophenol (4-NP) and electrochemical reduction of H2O2. The linear detection range and detection limit for the H2O2 sensor are estimated to be from 0.1 mM to 60 mM and 3.59 μM, respectively.
Fig. 1a shows the low magnification SEM image of the products thus formed (see ESI† for preparation details), which indicates that they consist exclusively of a large amount of submicrometre-scale particles. The high magnification SEM image reveals that these particles are spherical in shape and well-separated from each other, as shown in Fig. 1b. The TEM image further confirms the formation of colloidal spheres, as shown in Fig. 1c. A close view of one single sphere by TEM indicates that the resulting particles have an electron-microscopically perfect smooth surface (inset). Fig. 1d shows the corresponding particle size distribution histograms of these particles, which suggest that they have diameters ranging from 50 to 350 nm.
 | ||
| Fig. 1 (a) Low, (b) high magnification SEM, and (c) TEM images (inset: close view of one single particle) of the products thus formed; (d) shows the corresponding particle size distribution histograms. | ||
Fig. S1 (ESI†) shows the FTIR spectrum of the product. The peak at 3265 cm−1 can be attributed to the N–H stretching vibration. The peaks at 2918 and 2850 cm−1 are ascribed to the C–H stretching vibration. The major strong peaks at 1581 and 1511 cm−1 are ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the quinonoid and benzenoid rings, respectively. The peaks at 1445 and 1414 cm−1 can be ascribed to the C–H bending vibration. The peaks at 1300 and 1240 cm−1 are attributed to the C–N stretching vibration of the benzene ring. All these observations indicate that these particles are PANICSs.10
C stretching vibration of the quinonoid and benzenoid rings, respectively. The peaks at 1445 and 1414 cm−1 can be ascribed to the C–H bending vibration. The peaks at 1300 and 1240 cm−1 are attributed to the C–N stretching vibration of the benzene ring. All these observations indicate that these particles are PANICSs.10
Scheme 1 shows a schematic diagram to illustrate the formation mechanism of PANICSs by UV-assisted photopolymerization of aniline monomers using CND as a photocatalyst. The fluorescent CNDs have a strong UV absorption before 360 nm (Fig. S2, ESI†). Electron and hole pairs are generated in the CNDs upon UV excitation (reaction (1)).11 The photogenerated electrons are transferred to O2 in the solution generating O2− (reaction (2)).12 It is expected that aniline monomers can be adsorbed onto CNDs via π–π stacking interactions due to the π-rich nature of CNDs and subsequently oxidized by holes generated to produce PANI (reactions (3) and (4)).13 As control experiments, the oxidative polymerization reaction did not occur in the dark or without CNDs. All these observations indicate that CNDs serve as an effective photocatalyst for the photopolymerization of aniline monomers. It is well established that π-rich structures can quench fluorescence when fluorescent molecules are brought into close proximity to them.14 Given the π-rich nature of PANICSs, it is rational to believe that PANICSs can adsorb CNDs via π–π stacking interactions and quench fluorescence of CNDs. Indeed, substantial fluorescence quenching is observed for the CNDs–aniline mixture after UV irradiation (Fig. S3, ESI†). We believe that the CNDs are present on the surface of PANICSs and some are encapsulated in the PANICSs during their formation at the same time. However, because both CNDs and PANICSs share identical contrast under TEM observations, we cannot provide direct TEM evidence to support this. In addition, some CNDs should also be present in the dispersion, leading to the observation of residual fluorescence.
 | ||
| Scheme 1 A schematic diagram (not to scale) illustrating the formation proposed for PANICSs by UV-assisted photopolymerization of aniline monomers using CNDs as photocatalyst. | ||
The subsequent treatment of the PANICSs with an aqueous AgNO3 solution leads to spontaneous reduction of Ag+ ions to form AgNPs on PANICSs. Fig. 2a shows a typical TEM image of the PANICSs after their incubation with the aqueous AgNO3 solution over a 0.5 h period, which indicates that a large quantity of nanoparticles are generated on the PANICS surface. A close view of one single PANICS (Fig. 2b) further reveals that these nanoparticles are spherical in shape and range from 1 to 20 nm. The chemical composition of the products was further determined by the energy-dispersed spectrum, as shown in Fig. S4 (ESI†). The peaks of C, N and Ag elements are observed, indicating that the products are AgNPs-decorated PANICSs. The peaks of Cu element originate from the copper grid substrate used for TEM measurements. The HR-TEM image taken from one nanoparticle (inset in Fig. 2b) reveals clear lattice fringes with an interplanar distance of 0.235 nm corresponding to the (111) lattice space of metallic silver,15 providing another piece of evidence to support that the nanoparticles are AgNPs. Li et al. reported that poly(o-phenylenediamine) (POPD) microparticles can effectively adsorb Ag+ ions because many amino and imino groups located adjacent to each other in the POPD chains provide abundant coordination sites for Ag+ ions.16 Such an observation was further verified by the same group in an aromatic diamine polymers-based system.17 More recently, we have also demonstrated the in situ formation of AgNPs on POPD nanofibers and poly(m-phenylenediamine) structures.18 Similarly, the spontaneous formation of such AgNPs–PANICSs composites in our present study can also be attributed to the fact that PANICSs can effectively adsorb Ag+ ions via coordination of N atoms in PANI chains and then in situ reduce the as-adsorbed Ag+ ions to form AgNPs where the amino and imino groups in PANI serve as the electron donors.19
 | ||
| Fig. 2 (a) Low and (b) high magnification TEM images of PANICSs after treatment with an aqueous AgNO3 solution. Inset: a HR-TEM image of one single nanoparticle on PANICS. | ||
It is well known that 4-aminophenol (4-AP) is very useful and important in many applications that include analgesic and antipyretic drugs, photographic developers, corrosion inhibitors, anticorrosion lubricants, and so on.20 The reduction of 4-NP by NaBH4 in the presence of noble metal nanoparticles as catalysts has been intensively investigated for the efficient production of 4-AP.21 Therefore, the reduction of 4-NP to 4-AP with an excess amount of NaBH4 is used as a model system to quantitatively evaluate the catalytic activities of the AgNPs–PANICSs. As indicated in Fig. 3a, pure 4-NP shows a distinct spectral profile with an absorption maximum at 316 nm. When NaBH4 solution is added into the 4-NP, a new absorption peak is observed at 400 nm. The absorption intensity of 4-NP at 400 nm decreases quickly with time, accompanied by the appearance of the peak of 4-AP at 300 nm, indicating successful conversion of 4-NP to 4-AP. It should be noted that the reduction of 4-NP by NaBH4 is finished within 23 minutes upon the addition of AgNPs–PANICSs (Fig. 3b), with the observation of a fading and ultimate bleaching of the yellow-green color of the reaction mixture in aqueous solution. However, a longer reaction time of 72 minutes is required to achieve the full reduction using citrate-protected AgNPs as catalysts which contain equivalent Ag element (Fig. 3c). Without addition of a catalyst, the reduction will not proceed, and the absorption peak centered at 400 nm remains unaltered over time. However, due to the presence of a large excess of NaBH4 compared to 4-NP, the rate of reduction is independent of the concentration of NaBH4, and the reaction could be considered pseudo-first-order with respect to the concentration of 4-NP.22 Hence, ln(Ct/C0) versus time can be obtained based on the absorbance as a function of time, and good linear correlations are observed, as shown in Fig. 3d, suggesting that the reactions follow pseudo-first-order kinetics. Then, the kinetic reaction rate constants (defined as kapp) are estimated from the slopes of the linear relationship to be 5.17 × 10−2 min−1 (AgNPs) and 15.97 × 10−2 min−1 (AgNPs–PANICSs), respectively.
![UV–vis absorption spectra of 4-NP (a) and time dependent absorption spectra for the catalytic reduction of 4-NP by NaBH4 in the presence of AgNPs–PANICSs (b) and AgNPs (c). (d) Plot of ln(Ct/C0) of 4-NP against time for the catalysts. Conditions: [4-NP] = 3.5 mM; [Catalyst] = 15.27 mg L−1; [NaBH4] = 80 mM.](/image/article/2012/CY/c2cy00439a/c2cy00439a-f3.gif) | ||
| Fig. 3 UV–vis absorption spectra of 4-NP (a) and time dependent absorption spectra for the catalytic reduction of 4-NP by NaBH4 in the presence of AgNPs–PANICSs (b) and AgNPs (c). (d) Plot of ln(Ct/C0) of 4-NP against time for the catalysts. Conditions: [4-NP] = 3.5 mM; [Catalyst] = 15.27 mg L−1; [NaBH4] = 80 mM. | ||
These results clearly indicate that the AgNPs–PANICSs have higher catalytic activity for the reduction of 4-NP than the AgNPs only. The π-rich nature of the PANICSs support may play an active part in the catalysis, yielding a synergistic effect. The synergistically enhanced catalytic activity may be explained as follows: it is expected that 4-NP can be adsorbed onto PANICSs via π–π stacking interactions. Such adsorption provides a high concentration of 4-NP near to the AgNPs adsorbed on PANICS, leading to highly efficient contact between them. In contrast, without the presence of PANICSs, 4-NP must collide with AgNPs by chance and remain in contact for the catalysis to proceed. When this is not achieved, 4-NP will pass back into solution and can only react further when it collides with AgNPs again.23
H2O2 participates in a wide range of enzymatic reactions, playing an important role in the fields of chemistry, biology, clinical control and environmental protection, and therefore, its detection has attracted considerable research interest.24 We have developed an enzymeless H2O2 sensor based on AgNPs–PANICSs by deposition of the composites on a GCE surface (AgNPs–PANICSs/GCE). The PANICSs-modified GCE (PANICSs/GCE) was also similarly prepared. Fig. 4a shows the electrocatalytic responses of these electrodes toward the reduction of H2O2 in N2-saturated 0.2 M PBS at pH 6.5. In the presence of 1.0 mM H2O2, the AgNPs–PANICSs/GCE exhibits a remarkable catalytic reduction current peak at about 31 μA centered at −0.47 V vs. Ag/AgCl. However, the responses of both bare GCE and PANICSs/GCE toward the reduction of H2O2 are very weak. These observations indicate that the AgNPs supported on the PANICSs exhibit excellent catalytic performance toward H2O2 reduction, and the observation of large catalytic current could be attributed to the large amount of AgNPs existing on the surface of the PANICS. Fig. 4b shows a typical current–time plot of the AgNPs–PANICSs/GCE in N2-saturated 0.2 M PBS buffer (pH 6.5) on consecutive step change of H2O2 concentrations. When an aliquot of H2O2 was dropped into the PBS solution under stirring, the reduction current rose steeply to reach a stable value. The sensor could accomplish 95% of the steady state current within 3 s, indicating a fast amperometric response behavior. It is apparently seen that the steps showed in Fig. 4b are more horizontal in the region of lower concentration of H2O2 and the noises become higher with increased concentration of H2O2. The inset in Fig. 4b shows the calibration curve of the sensor. The linear detection range is estimated to be from 0.1 mM to 60 mM (r = 0.998), and the detection limit is estimated to be 3.59 μM at a signal-to-noise ratio of 3.
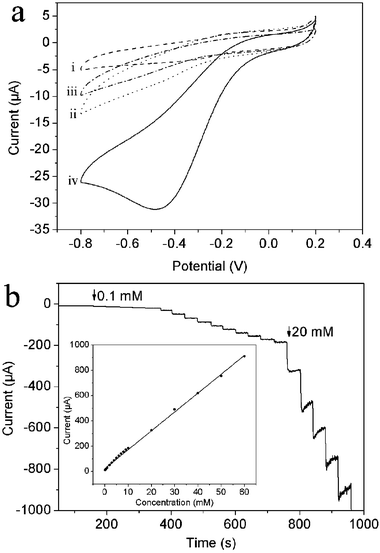 | ||
| Fig. 4 (a) Cyclic voltammograms of (i) bare GCE, (ii) PANICSs/GCE, (iii) AgNPs–PANICSs/GCE in N2-saturated 0.2 M PBS at pH 6.5 in the absence (iii) and presence (iv) of 1 mM H2O2 (scan rate: 50 mV s−1). (b) Typical steady-state response of the AgNPs–PANICSs/GCE to successive injection of H2O2 into the stirred N2-saturated 0.2 M PBS at pH 6.5. Inset: the corresponding calibration curve (applied potential: –0.3 V). | ||
In summary, fluorescent CNDs have been proven to be an effective photocatalyst for the photopolymerization of aniline monomers to form PANICSs. AgNPs-decorated PANICSs can be subsequently prepared by treating PANICSs with an aqueous AgNO3 solution. The resulting composites exhibit remarkable catalytic performance toward 4-NP reduction and the PANICSs support enhance the catalytic activity of Ag catalysts via a synergistic effect. Such composites also can effectively catalyze the electrochemical reduction of H2O2, leading to an enzymeless H2O2 sensor. Our present study is important because it provides us a general CNDs-based photopolymerization strategy toward conducting polymers for applications.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21175129) and the National Basic Research Program of China (No. 2011CB935800).Notes and references
- (a) C. L. Curtis, Adv. Mater., 1994, 6, 688 CrossRef; (b) A. G. MacDiarmid, Synth. Met., 1997, 84, 27 CrossRef CAS.
- (a) Q. Pei, G. Yu, C. Zhang, Y. Yang and A. G. Heeger, Science, 1995, 269, 1086 CAS; (b) A. G. Macdiarmid and J. A. Epstein, Synth. Met., 1994, 65, 103 CrossRef CAS; (c) X. Li, M. Huang, W. Duan and Y. Yang, Chem. Rev., 2002, 102, 2925 CrossRef CAS.
- (a) J. Huang and R. B. Kaner, J. Am. Chem. Soc., 2004, 126, 851 CrossRef CAS; (b) X. Zhang, W. J. Goux and S. K. Manohar, J. Am. Chem. Soc., 2004, 126, 4502 CrossRef CAS; (c) L. Liang, J. Liu, C. F. Windisch, G. J. Exarhos and Y. Lin, Angew. Chem., Int. Ed., 2002, 41, 3665 CrossRef CAS; (d) J. Huang and M. Wan, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1277 CrossRef CAS.
- (a) Z. Wei, Z. Zhang and M. Wan, Langmuir, 2002, 18, 917 CrossRef CAS; (b) Z. Zhang, Z. Wei and M. Wan, Macromolecules, 2002, 35, 5937 CrossRef CAS.
- A. D. W. Carswell, E. A. O'Rear and B. P. Grady, J. Am. Chem. Soc., 2003, 125, 14793 CrossRef CAS.
- Y. Tan, F. Bai, D. Wang, Q. Peng, X. Wang and Y. Li, Chem. Mater., 2007, 19, 5773 CrossRef CAS.
- (a) Y.-Z. Long, M.-M. Li, C. Gu, M. Wan, J.-L. Duvail, Z. Liu and Z. Fan, Prog. Polym. Sci., 2011, 36, 1415 CrossRef CAS; (b) Y. Wang, Z. Liu, B. Han, Z. Sun, Y. Huang and G. Yang, Langmuir, 2005, 21, 833 CrossRef CAS; (c) Z. Wei and M. Wan, Adv. Mater., 2003, 15, 136 CrossRef CAS; (d) H. Ding, M. Wan and Y. Wei, Adv. Mater., 2007, 19, 465 CrossRef CAS; (e) J. Li, H. Tang, A. Zhang, X. Shen and L. Zhu, Macromol. Rapid Commun., 2007, 28, 740 CrossRef CAS; (f) L. Huang, Z. Wang, H. Wang, X. Cheng, A. Mitra and Y. Yan, J. Mater. Chem., 2002, 12, 388 RSC; (g) S. Uemura, M. Yoshie, N. Kobayashi and T. Nakahira, Polym. J., 2000, 32, 987 CrossRef CAS.
- (a) L. Liu, D. Ma, H. Zheng, X. Li, M. Cheng and X. Bao, Microporous Mesoporous Mater., 2008, 110, 216 CrossRef CAS; (b) K. K. P. Datta, B. V. S. Reddy, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961 CrossRef CAS; (c) Y. Qiu and L. Gao, Chem. Commun., 2003, 2378 RSC; (d) A. Liu and M. Cohen, Science, 1989, 245, 841 CAS; (e) G. Yang and J. Wang, Appl. Phys. A: Mater. Sci. Process., 2000, 71, 343 CrossRef CAS; (f) Y. Bai, B. Lu, Z. Liu, L. Li, D. Cui, X. Xu and Q. Wang, J. Cryst. Growth, 2003, 247, 505 CrossRef CAS; (g) L. Yang, P. W. May, Y. Huang and L. Yin, J. Mater. Chem., 2007, 17, 1255 RSC.
- S. Liu, J. Tian, L. Wang, Y. Luo, J. Zhai and X. Sun, J. Mater. Chem., 2011, 21, 11726 RSC.
- J. Zhang, J. Tu, D. Zhang, Y. Qiao, X. Xia, X. Wang and C. Gu, J. Mater. Chem., 2011, 21, 17316 RSC.
- (a) G. Williams, B. Seger and P. V. Kamat, ACS Nano, 2008, 2, 1487 CrossRef CAS; (b) P. V. Kamat, I. Bedja and S. Hotchandani, J. Phys. Chem., 1994, 98, 9137 CrossRef CAS.
- R. Leary and A. Westwood, Carbon, 2011, 49, 741 CrossRef CAS.
- (a) S. Shukla, X. Vidal, E. P. Furlani, M. T. Swihart, K. T. Kim, Y. K. Yoon, A. Urbas and P. N. Prasad, ACS Nano, 2011, 5, 1947 CrossRef CAS; (b) M. Wang, Macromol. Rapid Commun., 2009, 30, 963 CrossRef.
- (a) B. Dubertret, M. Calame and A. J. Libchaber, Nat. Biotechnol., 2001, 19, 365 CrossRef CAS; (b) D. J. Maxwell, J. R. Taylor and S. Nie, J. Am. Chem. Soc., 2002, 124, 9606 CrossRef CAS.
- S. Navaladian, B. Viswanathan, T. K. Varadarajan and R. P. Viswanath, Nanotechnology, 2008, 19, 045603 CrossRef CAS.
- X. Li, X. Ma, J. Sun and M. Huang, Langmuir, 2009, 25, 1675 CrossRef CAS.
- X. Li, R. Liu and M. Huang, Chem. Mater., 2005, 17, 5411 CrossRef CAS.
- (a) J. Tian, S. Liu and X. Sun, Langmuir, 2010, 26, 15112 CrossRef CAS; (b) J. Tian, H. Li, W. Lu, Y. Luo, L. Wang and X. Sun, Analyst, 2011, 136, 1806 RSC; (c) J. Tian, Y. Luo, H. Li, W. Lu, G. Chang, X. Qin and X. Sun, Catal. Sci. Technol., 2011, 1, 1393 RSC.
- S. Ameen, M. S. Akhtar, Y. S. Kim, O.-B. Yang and H.-S. Shin, Colloid Polym. Sci., 2010, 289, 415 Search PubMed.
- (a) Y. Du, H. Chen, R. Chen and N. Xu, Appl. Catal., A, 2004, 277, 259 CrossRef CAS; (b) Z. Zhang, C. Shao, P. Zou, P. Zhang, M. Zhang, J. Mu, Z. Guo, X. Li, C. Wang and Y. Liu, Chem. Commun., 2011, 47, 3906 RSC.
- (a) Y. Deng, Y. Cai, Z. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8466 CrossRef CAS; (b) H. Jiang, T. Akita, T. Ishida, M. Haruta and Q. Xu, J. Am. Chem. Soc., 2011, 133, 1304 CrossRef CAS.
- J. Huang, S. Vongehr, S. Tang, H. Lu and X. Meng, J. Phys. Chem. C, 2010, 114, 15005 CAS.
- Y. Zhang, S. Liu, W. Lu, L. Wang, J. Tian and X. Sun, Catal. Sci. Technol., 2011, 1, 1142 CAS.
- (a) N. V. Klassen, D. Marchington and H. C. E. McGovan, Anal. Chem., 1994, 66, 2921 CrossRef CAS; (b) M. C. Y. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392 CrossRef CAS; (c) B. Wang, J. Zhang, Z. Pan, X. Tao and H. Wang, Biosens. Bioelectron., 2009, 24, 1141 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section; figures. See DOI: 10.1039/c2cy00439a |
| This journal is © The Royal Society of Chemistry 2012 |
