Effect of hydrocarbon tail-groups of transition metal alkoxide based amphiphilic catalysts on transesterification
Gayan
Nawaratna
,
Ronald
Lacey
and
Sandun D.
Fernando
*
Biological and Agricultural Engineering Department, Texas A&M University, USA. E-mail: sfernando@tamu.edu
First published on 8th November 2011
Abstract
In liquid/liquid/solid (L/L/S) systems pertinent to two immiscible reactant liquids mixed with a solid catalyst, the reaction efficacy depends on the mass transfer limitations at the L/L/S phase boundary. Formation of an emulsion in such a system will likely reduce the mass transfer barrier significantly. The stability of such an emulsion system depends on the hydrophilicity of the head group of the catalytic emulsifier toward the more polar liquid reactant and the hydrophobicity of the tail group toward the more nonpolar liquid reactant. This study looks at the effect of the alkyl groups with varying carbon numbers in titanium alkoxide as a catalyst that also has emulsification (amphiphilic) properties to transesterify triglycerides in alcohols. All forms of oligomeric titanium alkoxides tested were highly basic. Those with smaller alkoxide groups (lower carbon numbers) tended to be more basic than those with higher carbon numbers. The chirality did not affect the degree of basicity of the alkoxides. The maximum ester yield noticed was 64.25% (with 63.85% selectivity towards transesterification) with titanium methoxide after 3 hours of reaction. It was observed that higher the number of carbon atoms in the tail group the lower the catalytic ability of the amphiphile towards transesterification. It is expected that longer the carbon-chain in the tail group stronger the emulsification ability of the amphiphile in oil-in-alcohol systems. However, when looking at the efficacy of the amphiphile for the combined emulsification and catalytic ability, it is apparent that the length of the alkoxide group needs to be compromised.
Introduction
Background
In reaction engineering, there are numerous instances where two immiscible liquid reactants (such as polar and non-polar liquid reactants) are brought in contact with a solid catalyst (i.e. liquid/liquid/solid (L/L/S) immiscible system). Such systems that use heterogeneous catalysts include esterification,1,2 transesterification,3–6 etherification,7,8 and hydrolysis.9–11 The effectiveness of the reaction in such systems is gravely limited by mass transfer issues associated with immiscible phases as well as the unavailability of sufficient interfacial area. Although the L/L incompatibility could be ameliorated by introducing a liquid surfactant, this causes downstream product separation problems waning the effectiveness of such a system for practical applications.12 The primary goal of this study is to address these limitations by developing a heterogeneous (solid) catalyst that acts as an emulsifier (amphiphile) which essentially will be positioned at the interface between the two immiscible liquids while also catalyzing the reaction.13 It was conceptualized that the amphiphilic catalyst first brings the two hydrophilic and hydrophobic liquid molecules together, and due to the emulsification properties of the catalyst stabilizes the emulsion. In the mean time, the catalyst will lend its active sites for the desired reaction to occur.In a previous study, the effectiveness of titanium isopropoxide based monomers, oligomers and polymers as transesterification catalysts was studied.13 It was observed that there was an optimal level of oligomerization that is most effective in transesterification catalysis. An aforementioned study looked at the catalytic ability of methoxide groups that are tethered to the Ti–O–Ti matrix of different polymeric complexities. However, the study did not look at the behavior of such a system to alkoxide groups with varying carbon composition. The importance of the carbon composition is that in alcohol-in-oil systems where oil is the continuous phase, the longer the hydrophobic tail the better the ability of the amphiphile to stabilize an alcohol-in-oil emulsion. The objective of this study is to fill this gap, i.e., understand the catalytic behavior of titanium based amphiphiles with alkoxide groups with varying carbon numbers (in terms of length and enantiomers).
We selected transesterification reaction to test our premise due to a multitude of reasons. This reaction has gained much attention recently due to its use in the biodiesel industry.14,15Fatty acid methyl (or ethyl) esters, commonly known as biodiesel, are a renewable alternative fuel for compression ignition engines.16–21 Typical raw materials used are triglycerides of either plant or animal origin.20 The reaction is ideal since the reactants (triglyceride and alcohol) are immiscible in each other. Moreover, since the reaction has been widely studied, there is enough bench mark data to compare the effectiveness of heterogeneous amphiphilic catalysts on which we are working.
Transesterification is an acid or alkaline catalyzed reaction.22 Due to superior activity and favorable economics, the most commonly used industrial catalysts are sodium and potassium hydroxides that are in a homogeneous phase as the reactants. However, alkaline hydroxides often produce saponifiable matter23 which originates from the free fatty acid neutralization. The soap formation is undesirable as it partially consumes the catalyst, decreases biodiesel yield, and complicates the separation and purification steps. In addition, the removal of these homogeneous catalysts is technically difficult and adds extra cost to the final products.24–26 Moreover, disposal of the catalyst-contaminated glycerin is increasingly becoming an environmental concern. Therefore, heterogeneous catalysis is desired to simplify separation and purification of the products. Development of a heterogeneous catalyst that also helps ameliorate transport limitations would be an added potential benefit of this study to the biodiesel industry.
Selection of titanium as the base metal and its alkoxides as the active site with amphiphilic properties was based on several reasons. Titanium based alkoxides (especially titanium isopropoxide) are a widely used reagent in sol–gel chemistry27 and chemical vapor deposition.27,28 As a result, the chemistry of primary alkoxides is well characterized and understood. Also, titanium based alkoxides have already been successfully used for transesterification in previous studies.13
Materials and methods
Materials
The transesterification reaction was carried out in a high pressure thermal reactor (4570-Parr Instrument, Moline, IL, USA) with a maximum operating temperature of 500 °C and a pressure of 5000 psi. The reactor was used in the batch mode. Degummed soybean oil was purchased in bulk from STE Oil Company, San Marcos, TX, USA.Catalysts, titanium methoxide, titanium ethoxide, titanium propoxide, titanium isopropoxide, and titanium butoxide, were purchased from Sigma-Aldrich Chemical Company. Titanium isobutoxide was purchased from Alfa Aesar Company. In terms of alcohols, isopropanol was purchased from EMD Chemicals Inc. Propanol and butanol were purchased from Sigma-Aldrich Chemical Company while ethanol and methanol were purchased from VWR International LLC. Isobutanol was purchased from Alfa Aesar Company. Pure biodiesel was purchased from SoyGold (Ag Environmental Products, LLC, Omaha, NE, USA). Hammett indicators 2,4-dinitroaniline, 4-chloro-2-nitroaniline, 4-chloroaniline, crystal violet, dimethyl yellow, methyl red, neutral red, Nile blue, phenolphthalein and tropaeolin were purchased from Sigma-Aldrich Chemical Company.
Catalyst preparation
All the catalysts were prepared by ultrasonic mixing with respective alcohols. Titanium methoxide based oligomer catalysts were prepared using ultrasonic mixing of titanium methoxide with methanol and then prescribed amounts of water. The sonication system (UP400S, Hielscher Ultrasound Technology) is capable of producing 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kHz waves with a power output of 400 W for this purpose.
000 kHz waves with a power output of 400 W for this purpose.
The catalyst polymerization was kept at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 alkoxide
0.5 alkoxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mole ratio13 by controlling the degree of polymerizationviawater condensation. Although adding stoichiometric amounts of water and alkoxide, this will not guarantee the hydrolysis of half of the alkoxides.29–33 Adding limited water will limit the hydrolysis of alkoxides which in turn will limit the particle size. It has been studied that alkoxides with larger tail groups hydrolyze and diffuse slowly. Because of that large tail groups alkoxides tend to make smaller polymeric particles.33 Dilution of water and alkoxide in a solvent affects the mean particle size of the product obtained from hydrolysis and polymerization.34 The water needed for condensation–polymerization was first diluted in the respective alcohol prior to the addition into the titanium alkoxide monomer under ultrasonication. Dilution of water in alcohol makes the water well dispersed and in turn helps in formation of a polymer with consistent molecular size. Although the probable end products obtained from the hydrolysis and condensation of alkoxide are oligomers, we have given the basic dimerization reaction here for basic understanding of the reaction. An example of condensation dimerization reaction for titanium isopropoxide is given in Fig. 1.
water mole ratio13 by controlling the degree of polymerizationviawater condensation. Although adding stoichiometric amounts of water and alkoxide, this will not guarantee the hydrolysis of half of the alkoxides.29–33 Adding limited water will limit the hydrolysis of alkoxides which in turn will limit the particle size. It has been studied that alkoxides with larger tail groups hydrolyze and diffuse slowly. Because of that large tail groups alkoxides tend to make smaller polymeric particles.33 Dilution of water and alkoxide in a solvent affects the mean particle size of the product obtained from hydrolysis and polymerization.34 The water needed for condensation–polymerization was first diluted in the respective alcohol prior to the addition into the titanium alkoxide monomer under ultrasonication. Dilution of water in alcohol makes the water well dispersed and in turn helps in formation of a polymer with consistent molecular size. Although the probable end products obtained from the hydrolysis and condensation of alkoxide are oligomers, we have given the basic dimerization reaction here for basic understanding of the reaction. An example of condensation dimerization reaction for titanium isopropoxide is given in Fig. 1.
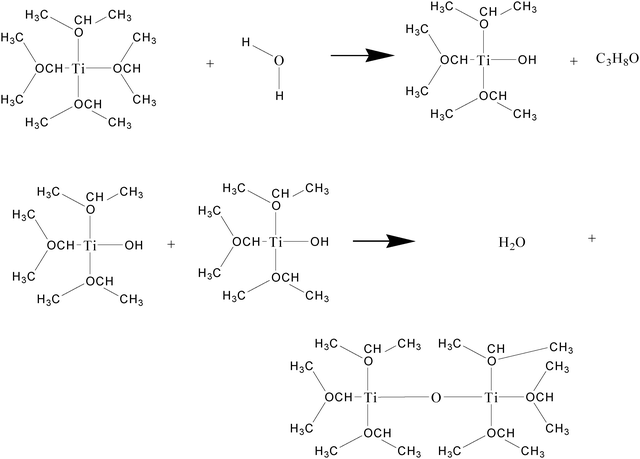 | ||
| Fig. 1 Hydrolysis and water condensation reactions of titanium isopropoxide. | ||
In this reaction, first, a water molecule hydrolyzes an alkoxide bond of the titanium isopropoxide generating a hydroxyl group. Two such molecules having orthogonal hydroxyl groups react to give –Ti–O–Ti– viawater condensation. The degree of polymerization of metal alkoxides could be controlled by changing the alkoxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratios in a similar fashion. The starting monomers and resulting oligomers are given in Table 1.
water ratios in a similar fashion. The starting monomers and resulting oligomers are given in Table 1.
| Monomer | Resulting oligomer |
|---|---|
| Titanium methoxide |

|
| Titanium ethoxide |

|
| Titanium n-propoxide |

|
| Titanium iso-propoxide |

|
| Titanium n-butoxide |

|
| Titanium iso-butoxide |
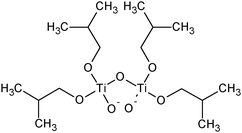
|
Transesterification
The transesterification reactions were carried out in a high pressure reactor with a vessel of 500 ml. The pressure reactor consists of a magnetic drive stirrer with a maximum speed of 2000 rpm (tachometer module with accuracy ± 10 rpm). In order to initiate the transesterification reaction, 1% (w/w) of the catalyst was infused into the reaction chamber containing triglyceride, immediately after the contents have reached the designated temperature (200 °C) through a high pressure liquid pump (Eldex 5790, Eldex Laboratories Inc., Napa, CA). It was noticed that when the reactor reached 200 °C, the pressure increased up to 10 psi. Samples were drawn from the reactor at 30 minute intervals up to 3 hours. The samples drawn were cooled immediately in order to cease the reaction from progressing further. Then, the samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 12 °C to separate the products (alkyl esters and glycerol) and the catalyst. The centrifuge system used (Sorvall Legend 23 R- Thermo Scientific) has a maximum rated speed of 24
000 rpm at 12 °C to separate the products (alkyl esters and glycerol) and the catalyst. The centrifuge system used (Sorvall Legend 23 R- Thermo Scientific) has a maximum rated speed of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and a minimum temperature of (−)4 °C.
000 rpm and a minimum temperature of (−)4 °C.
The top fraction of the products was analyzed for esters using gas chromatography (GC-6850 Agilent Technologies, Santa Clara, CA, USA). Auxiliary analyses for confirmation of the products were carried out viaGC Mass Spectroscopy (GC-MS 7890 Agilent Technologies, Santa Clara, CA, USA).
The gas chromatograph was calibrated with the respective alkyl ester standards each time prior to obtaining quantitative yields. Alkyl ester standards (pertinent to methyl, ethyl, propyl, isopropyl, butyl, and isobutyl esters of C 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 18
0, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 18
0, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 18
1, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 18
2, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 fatty acid alkyl esters) were purchased from Nu-Chek Prep Inc. (Elysian, MN 56028, USA).
3 fatty acid alkyl esters) were purchased from Nu-Chek Prep Inc. (Elysian, MN 56028, USA).
The GC method utilized (for isopropyl esters) detection is given below:
| Inlet temperature | 250 °C |
| Split ratio | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Injection volume | 1 μl |
| Column flow (helium) | 1.6 ml min−1 (constant flow) |
| FID temperature | 280 °C |
| H2 flow | 40 ml min−1 |
| Air flow | 450 ml min−1 |
| Makeup gas (nitrogen) | 30 ml min−1 |
| Oven program | 50 °C hold 1 min, to 200 °C at 25 °C min−1, hold 3 min, to 230 °C at 3 °C min−1, hold 18 min |
| Column | 30 m × 0.25 mm × 0.25 μm (DB-Wax Column) |
The fatty acid alkyl esters yield was calculated by Chemstation software (Agilent Technologies). An internal standard method was used to analyze the ester yields. The area under the peak from the FID chromatogram corresponded to the concentration of that component. These concentrations were determined using calibrations with pure ester standards along with an internal standard (C-12 ester).
Catalyst selectivity
There are many definitions for calculating selectivity in the literature. IUPAC defines selectivity in two ways: (1) the discrimination shown by a given reactant A when it reacts with two alternative reactants B and C, or in two different ways (e.g. at two different sites) with a reactant B and (2) the ratio of products obtained from given reactants. In the context of this work, the following formula was used:35The results were statistically analyzed by Design Expert software.
Catalyst characterization
The basic strength of the catalyst was determined by the Hammett indicator method.36 The Hammett indicator method is used to determine qualitative information of the basic properties of solid catalysts. The Hammett indicator method is a fast and straightforward method to analyze the basicity and acidity of a catalyst. This method is only accurate for qualitative analysis because of potential issues associated with indicator molecules diffusing into the micropores.36,37 Nevertheless, the basicities obtained by using the Hammett indicator method are in good agreement with the catalytic findings.About 10 ml of sample containing catalyst was shaken with 5 drops of Hammett indicator in methanol solution and left to equilibrate for 3 h. In the Hammett indicator method, the base strength is quoted as being stronger than the weakest indicator which exhibits a color change, but weaker than the strongest indicator that produces no color change.36,37 In these experiments, the following Hammett indicators were used: neutral red (pKa, 6.8), methyl red (pKa, 4.8), p-dimethylaminoazobenzene (pKa, 3.3), and crystal violet (pKa, 0.8). The acidic Hammett indicators (for base site strength) used were phenolphthalein (pKBH+, 8.2), Nile blue (pKBH+, 10.1), tropaeolin (pKBH+, 11), 2,4-dinitroaniline (pKBH+, 15), 4-chloro-2-nitroaniline (pKBH+, 18.2), and 4-chloroaniline (pKBH+, 26.5). To measure the basicity of the catalysts, the method of Hammett indicator–benzene carboxylic acid (0.02 mol l−1 anhydrous methanol solution) titration was used.20–22
Results and discussion
The primary goal of this study was to understand the link behind the emulsifying ability of a molecule and its catalytic properties. Although this experiment did not measure the emulsification ability of the selected catalysts, it is an established fact that molecules having longer hydrophobic tails (with a hydrophilic head) will have better emulsifying ability in water-in-oil emulsions.38 Analogously, it was conjectured that the catalysts with longer tail-groups will have better emulsifying abilities in alcohol-in-oil emulsions. The catalytic behavior of the selected amphiphiles is analyzed below with this premise in mind.Fig. 2 shows the respective ester yields with tail groups of increasing carbon numbers. The selected cubic model response surface indicates that, irrespective of the reaction time, ester yields tend to decrease with increasing carbon number on the tail. The maximum reported ester yield was 64.25% and this was when titanium methoxide was used as the catalyst.
 | ||
| Fig. 2 Ester yield variation with respect to amphiphiles with different carbon numbers. Note—graph on right depicts the ester yield trend line (solid line) and ± error (variance) in dotted lines. | ||
The reason for smaller alcohols to display significantly higher catalytic ability may be due to favorable mass transport properties that smaller molecules have as compared to larger counterparts. Smaller methoxide molecules may be more mobile between phases assisting catalysis within the L/L/S system.
As expected, the ester yields increased with increase in reaction time. The statistical analysis points out that although the two factors (time and carbon number) were significant, their interaction was not significant.
The graph on the right side of Fig. 2 depicts ester yield at 3 hours (along with the (±) standard deviation lines) vs. the carbon number. It is interesting to note that a clear increase in esters yield is observable when butoxide is used instead of the shorter form, propoxide. The likely scenario in this instance would be the further reduction of transport limitations of the alcohol/oil system resulting from the increase of hydrophobicity of the alcohol. In such an instance, all three components (i.e.alcohol, oil and the catalyst) would be substantially hydrophobic. Alternatively, the alkyl groups in simple alcohols may not be large enough to generate such hydrophobicity for causing such a phenomenon.
The catalyst selectivity is depicted in Fig. 3. It could be noted that the selectivity drastically decreases with the increase in number of carbons in the tail group. Here it could be seen that the selectivity trend does not necessarily follow the yield trends, especially when it comes to higher carbon-numbered alkoxides.
 | ||
| Fig. 3 Ester selectivity with respect to different carbon number tail groups and time. | ||
Fig. 3 (right) shows selectivity variation with (±) standard deviations after the reaction was performed for 3 hours. It is clear that the selectivity reduces linearly as a function of number of carbons in the tail group. The maximum selectivity noticed was 63.85 with titanium methoxide after 3 hours of reaction. Also, referring Fig. 3 (left) it appears like although the reactions with smaller alcohols have not reached equilibrium even after three hours; those with large alcohols had reached equilibrium by that time. Both these observations confirm the explanations given for the higher catalytic performance of smaller alkoxides as described earlier.
Fig. 4 depicts the yield differentiation between isomers with longer tail groups (propoxide and butoxide). In this case, the objective is to discern the catalytic variations of alkoxides due to linear and branched tail groups. Titanium isopropoxide and titanium isobutoxide were used against titanium propoxide and titanium butoxide to analyze the stereo-effects.
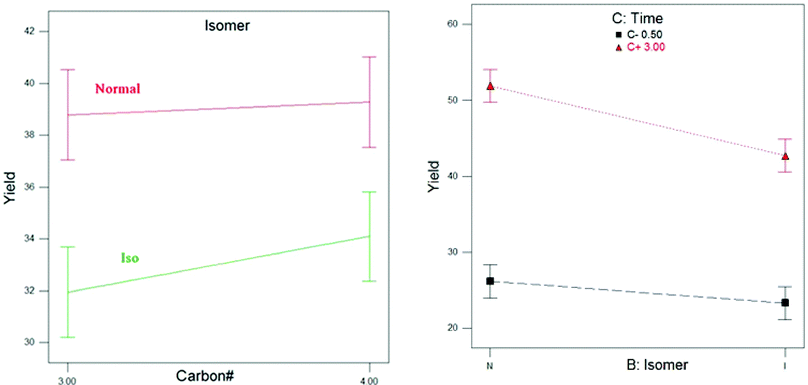 | ||
| Fig. 4 Ester yield with respect to different isomer types (for carbon numbers 3 and 4 only). | ||
Fig. 4 (left) shows how the carbon number affects the ester yields. The analysis depicts that the ester yields do not differ significantly between propoxides and butoxides (although statistically not relevant, a clear yield increase is present when going from 3C to 4C alkoxides). However, the normal and iso forms significantly affect the ester yields.
According to Fig. 4 (right), it is observed that the ester yields do not depend on the type of isomer at lower temperatures. However, as the temperature is increased, the normal alkoxides display a much profound activity than the iso-forms. The likely reason for this is the steric hindrance.
Catalyst characterization
Catalyst basicity using the Hammett indicator method is depicted in Table 2. Based on the observations, the smaller forms, titanium methoxide and titanium ethoxide, had a pKa value of 11.2 while the more larger forms, titanium butoxide and titanium propoxide, had a pKa value of 10.1. The basicity analysis indicates that the alkoxides with higher basicity resulted in more ester yields indicating that the shorter forms are more prone to ionization in the medium as compared to the more bulky alkoxides. Since there was no significant color change between iso and normal alkoxides, it was conjectured that these forms had the same basicity.| Catalyst | pKa value (Hammett indicator method) |
|---|---|
| Titanium methoxide oligomer | 11.2 |
| Titanium ethoxide oligomer | 11.2 |
| Titanium propoxide oligomer | 10.1 |
| Titanium isopropoxide oligomer | 10.1 |
| Titanium butoxide oligomer | 10.1 |
| Titanium isobutoxide oligomer | 10.1 |
Based on the above observations, the transesterification reaction mechanism given in Scheme 1 is initially proposed.39
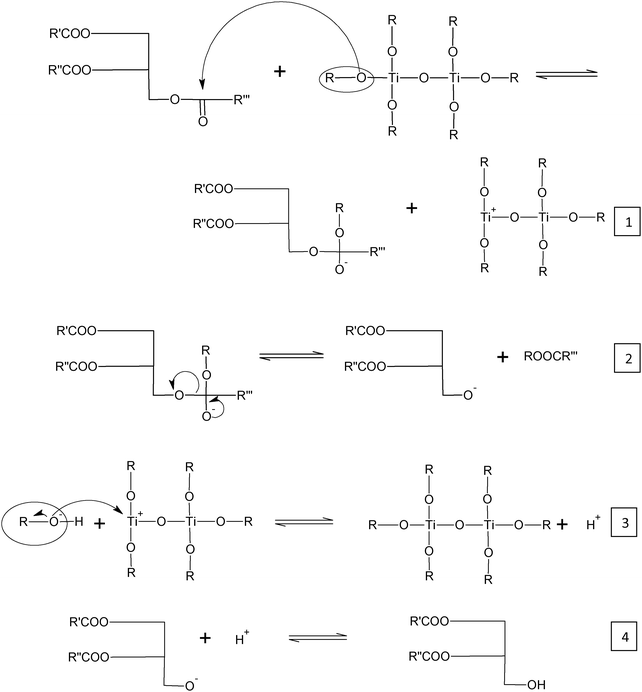 | ||
| Scheme 1 Proposed mechanism for metal alkoxide transesterification of soybean oil. | ||
In the first step, titanium alkoxide will initiate the reaction by nucleophilic attack on the carbonyl group in the triglyceride (eqn (1)). This will create an intermediate (tetrahedral) which will produce an alkyl ester and a diglyceride nucleophile (eqn (2)) and a positively charged catalyst. The now electrophilic titanium catalyst will get attacked by the respective alcohol while the alcohol getting deprotonated (eqn (3)). This will create an extra proton which will terminate the reaction by producing a molecule of diglyceride and a fatty acid alkyl ester (eqn (4)). The reaction continues until glycerol and three molecules of fatty acid alkyl esters are produced. This scheme is proposed based on the Brønsted basicity that the titanium alkoxides displayed (similar to main group metal alkoxides)39 in which alkoxides of the outer-sphere migrate and attack the ester bond of the triglyceride.
However, another possibility is that the transesterification is a concerted process occurring at the Ti center. In this premise, there is no migration of the alkoxide group from the titanium to the ester resulting in a coordinately unsaturated titanium cation and an organic anion such as is shown in reaction (1) under Scheme 1. It is likely that both of those species are thermodynamically unstable and would not form as discrete species in solution. Ti(IV) is a high valent metal and, as such, is a strong Lewis acid with vacant d orbitals to accommodate the lone pair from the ester (triglyceride). In this case, the triglyceride ester coordinates directly with the Ti and group migration occurs concertedly at the metal center, ultimately eliminating the new ester. This premise is proposed since there is never a time when any of the ionic species shown in the mechanistic scheme would exist in solution. Accordingly, the reaction mechanism in Scheme 2 is proposed.
 | ||
| Scheme 2 Alternative mechanism for metal alkoxide transesterification of soybean oil. | ||
In the first step, titanium alkoxide will initiate the reaction by nucleophilic attack on the carbonyl group in the triglyceride (eqn (1)). Here titanium isopropoxide acts as a Lewis acid, and the non-bonding electrons on the alkoxide oxygen forms a coordinate bond with empty d orbitals on the metal.40 This will create a coordinated intermediate (tetrahedral) which will produce an alkyl ester and a diglyceride nucleophile (eqn (2)). Then the electrophilic titanium catalyst will get attacked by the respective alcohol while the alcohol getting deprotonated (eqn (3)). The reaction continues until glycerol and three molecules of fatty acid alkyl esters are produced.40
The mechanism proposed in Scheme 2 also gives a clue as to why the smaller Ti alkoxides are more reactive. It is likely that the sterically smaller alkoxides allow the ester to coordinate more easily to the metal. They also provide a much smaller barrier to group migration around the metal to achieve transesterification.
Conclusion
This study looked at the catalytic behavior of oligomerized alkoxides with varying carbon numbers. The study confirmed that the number of carbons in alkoxides and their steric effects significantly affect the ester yields and selectivity in transesterification. The maximum ester yield (64.25%) and selectivity (63.85%) obtained were with oligomerized titanium methoxide catalyst after 3 hours of reaction. Although the temperature and the number of carbon in the tail group significantly affect the ester yields, a statistically significant correlation was not present (the two parameters affected ester yields independently). It was clear that the alkoxides with a smaller carbon numbers had a superior selectivity towards transesterification. It appears that the sterically smaller alkoxides allow the ester to coordinate more easily to the metal. They also provide a much smaller barrier to group migration around the metal to achieve transesterification. An interesting observation was the slight increase of esters yield when butoxides were used as the catalyst as opposed to propoxides. This behavior was attributed to the overall increase of the hydrophobicity of the three-component system.References
- H. Kim, B. Kang, M. Kim, D. Kim, J. Lee and K. Lee, Stud. Surf. Sci. Catal., 2004, 153, 4 Search PubMed.
- F. T. Sejidov, Y. Mansoori and N. Goodarzi, J. Mol. Catal. A: Chem., 2005, 240, 186 CAS.
- J. F. Puna, J. F. Gomes, M. J. N. Correia, A. P. Soares Dias and J. C. Bordado, Fuel, 2010, 89, 3602 CrossRef CAS.
- J. Gomes, J. Puna, J. Bordado and M. Correia, React. Kinet. Catal. Lett., 2008, 95, 273 CrossRef CAS.
- S. Benjapornkulaphong, C. Ngamcharussrivichai and K. Bunyakiat, Chem. Eng. J., 2009, 145, 468 CrossRef CAS.
- H. Chen, B. Peng, D. Wang and J. Wang, Front. Chem. Eng. China, 2007, 1, 11 CrossRef.
- G. D. Yadav and M. S. Krishnan, Ind. Eng. Chem. Res., 1998, 37, 3358 CrossRef CAS.
- J. W. Kim, D. J. Kim, J. U. Han, M. Kang, J. M. Kim and J. E. Yie, Catal. Today, 2003, 87, 195 CrossRef CAS.
- O. Kröcher and M. Elsener, Appl. Catal., B, 2009, 92, 75 CrossRef.
- H. Iloukhani, S. Azizian and N. Samadani, React. Kinet. Catal. Lett., 2001, 72, 239 CrossRef CAS.
- V. Lykourinou-Tibbs, A. Ercan and L.-J. Ming, Catal. Commun., 2003, 4, 549 CrossRef CAS.
- A. Nandi, A. Mehra and D. V. Khakhar, Fluid Mechanics and Transport Phenomena, 2005, 52, 10 Search PubMed.
- G. Nawaratna, S. D. Fernando and S. Adhikari, Energy Fuels, 2010, 24, 4123 CrossRef CAS.
- Y. Zhang, M. A. Dube, D. D. McLean and M. Kates, Bioresour. Technol., 2003, 89, 16 CrossRef.
- R. Bray, Biodiesel via Axens Heterogeneous Catalysis Esterfip-H Process, SRI Consulting, 2005 Search PubMed.
- J. V. Gerpen, B. Shanks and R. Pruszko, Biodiesel Production Technology, 2004 Search PubMed.
- A. C. Pinto, L. L. N. Guarieiro, M. J. C. Rezende, N. M. Ribeiro, E. A. Torres, W. A. Lopes, P. A. P. Pereira and J. B. Andrade, J. Braz. Chem. Soc., 2005, 16, 18 Search PubMed.
- J. Hancsok, F. Kovacs and M. Krar, Pet. Coal, 2004, 46, 9 Search PubMed.
- J. Rothermel, Iowa State University, 2003.
- M. Zappi, R. Hernandez, D. Sparks, J. Horne, M. Brough, S. M. Arora and W. D. Motsenbocker, A Review of the Engineering Aspects of the Biodiesel Industry, MSU E-TECH Laboratory, 2003 Search PubMed.
- J. V. Gerpen, B. Shanks, R. Pruszko, D. Clements and G. Knothe, Biodiesel Analytical Methods, NREL, 2004 Search PubMed.
- B. Wenzel, M. Tait, A. Modenes and A. Kroumov, Bioautomation, 2006, 5, 10 Search PubMed.
- W. Zhou and D. G. B. Boocok, J. Am. Oil Chem. Soc., 2006, 83, 6 Search PubMed.
- J. V. Thompson and B. B. He, Appl. Eng. Agric., 2006, 22, 5 Search PubMed.
- N. Pachauri and B. He, In 2006 ASABE Annual International Meeting, Portland, Oregon, 2006 Search PubMed.
- H. Noureddini, W. R. Dailey and B. A. Hunt, Adv. Environ. Res., 1998, 2, 12 Search PubMed.
- D. C. Bradley, Chem. Rev., 1989, 89, 1317 CrossRef CAS.
- M. Ritala, M. Leskela, L. Niinisto and P. Haussalo, Chem. Mater., 1993, 5, 1174 CrossRef CAS.
- C. F. Campana, Y. Chen, V. W. Day, W. G. Klemperer and R. A. Sparks, J. Chem. Soc., Dalton Trans., 1996, 691 RSC.
- B. E. Yoldas, J. Non-Cryst. Solids, 1982, 51, 105 CrossRef CAS.
- B. E. Yoldas, J. Non-Cryst. Solids, 1980, 38–39(Part 1), 81 CrossRef CAS.
- B. E. Yoldas, J. Non-Cryst. Solids, 1984, 63, 145 CrossRef CAS.
- B. E. Yoldas, J. Mater. Sci., 1986, 21, 1087 CrossRef CAS.
- B. E. Yoldas, J. Am. Ceram. Soc., 1982, 65, 387 CrossRef CAS.
- D. Ferdous, A. K. Dalai and J. Adjaye, Fuel, 2006, 85, 1286 CrossRef CAS.
- L. P. Hammett and M. A. Paul, J. Am. Chem. Soc., 1934, 56, 827 CrossRef CAS.
- W. Xie and X. Huang, Catal. Lett., 2006, 107, 53 CrossRef CAS.
- L. E. Orgel, An introduction to transition-metal chemistry ligand-field theory, 1 edn, John Wiley & Sons Inc., New York, 1960 Search PubMed.
- U. Schuchardt, R. Sercheli and R. M. Vargas, J. Braz. Chem. Soc., 1998, 9, 199 CrossRef CAS.
- M. D. Curran, T. E. Gedris and A. E. Stiegman, Chem. Mater., 1998, 10, 1604 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |

