PS-Pd–NHC: an efficient and heterogeneous recyclable catalyst for direct reductive amination of carbonyl compounds with primary/secondary amines in aqueous medium†
Dattatraya B.
Bagal
,
Rahul A.
Watile
,
Mayur V.
Khedkar
,
Kishor P.
Dhake
and
Bhalchandra M.
Bhanage
*
Department of Chemistry, Institute of Chemical Technology, Matunga, Mumbai 400 019, India. E-mail: bm.bhanage@gmail.com; bm.bhanage@ictmumbai.edu.in; Fax: +91-22 3361 1020; Tel: +91-22 3361 1111/222
First published on 3rd November 2011
Abstract
A highly efficient polymer supported palladium-N–heterocyclic carbene (PS-Pd–NHC) catalytic system has been developed for direct reductive amination (DRA) of carbonyl compounds with primary/secondary amines in aqueous reaction medium. This new catalytic system represents a heterogeneous, recyclable and environmentally benign protocol. The developed methodology describes a simple one step approach for the synthesis of a wide variety of substituted amines exhibiting remarkable activity with excellent yield of a desired product. Furthermore, the catalyst was effectively recycled for six consecutive cycles without any significant loss in its catalytic activity.
Introduction
Amines are important building blocks possessing wide application in agrochemicals, fine chemical industries, pharmaceuticals, material science and biotechnology.1,2 Direct reductive amination (DRA) of carbonyl compounds with amines is one of the most useful methods for one step synthesis of secondary or tertiary amines and related functional compounds.3,4 For this transformation various boron-based reagents have been developed including borane–pyridine,5sodium borohydride–magnesium perchlorate,6ZnBH4–ZnCl2,7NaBH(OAc)3,8silica gel–ZnBH4,9dibutyl tin chloride hydride,10NaBH3CN11 and InCl3/Et3SiH.12 However, these systems require the use of a stoichiometric amount of hydride source, excess amine and acidic reaction conditions. Some recent approaches include use of an organotin catalyst for reductive amination by phenylsilane, poly(methylhydrosiloxane PMHS),13 transition metal catalyzed direct reductive amination by a transfer hydrogenation method14 and some heterogeneous palladium catalysts.15 Despite their potential utility, the reported protocols suffer from one or more drawbacks such as use of acidic conditions, poor stability, toxic by-products, which carries the risk of having residual cyanide content in the synthesized product, use of pyridine–borane which is thermally unstable and has to be handled with extreme care, use of hazardous organic solvents, and use of expensive ligands and homogeneous non-recyclable catalytic system with difficulties in catalyst–product separation, thereby limiting their general applications. Therefore, the development of newly improved protocols for performing direct reductive amination by using greener and milder reaction conditions is still desirable.In recent decades, use of water as a reaction medium for transition metal-catalyzed reactions has merited increasing attention and is currently one of the most important challenges for development of sustainable green chemistry.16Water, an inexpensive, safe, readily available and non-toxic solvent provides remarkable advantages over common organic solvents from economic and environmental points of view.17 Hence, development of catalytic protocols employing water as a reaction medium could be the answer for the future of green chemistry.
Recently, N-heterocyclic carbenes (NHCs) have been found to become valuable ligands in organometallic chemistry and catalysis18 because of their effective binding ability to any transition metal irrespective of their oxidation states. Palladium–N-heterocyclic carbene (Pd–NHC) systems have been successfully employed in various reactions such as C–C and C–N coupling reactions, oxidation reactions19 and more recently a heterogeneous palladium–NHC system has been explored for hydrogenation reactions.20 The heterogeneous PS-Pd–NHC complex offers several advantages like reuse of expensive transition metals and ligands with a possibility to prevent the contamination of ligand residue in synthesized products.
In continuation with our ongoing research on development of a new facile protocol for direct reductive amination21 we herein report a simple, efficient, greener and recyclable approach for direct reductive amination of carbonyl compounds with primary and secondary amines by using PS-Pd–NHC as a versatile catalyst in aqueous media (Scheme 1). However, to the best of our knowledge, no such polymer supported heterogeneous palladium–NHC catalytic system has been yet explored for the synthesis of a wide variety of amines using a direct reductive amination reaction.
 | ||
| Scheme 1 Direct reductive amination of carbonyl compounds with primary/secondary amines. | ||
Results and discussion
To optimize the reaction conditions, the reaction of benzaldehyde with aniline in the presence of PS-Pd–NHC as a catalyst was chosen as a model reaction for direct reductive amination. Various reaction parameters such as catalyst loading, effect of solvent, effect of hydrogen pressure, reaction temperature and time were studied and the results obtained are summarized in Tables 1 and 2. Initially we studied the effect of catalyst loading ranging from 0.10 mol% to 0.20 mol%, where increase in the initial catalyst concentration from 0.10 to 0.15 mol% has increased the yield of the desired product (Table 1, entries 1 and 2) while further increase in the amount of catalyst (0.20%) had no profound effect on the yield of the desired product (Table 1, entry 3).| Entry | Solvent | H2/bar | Temperature/°C | Time/h | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: benzaldehyde, 6 mmol; aniline, 5 mmol; catalyst, 0.15 mol%; water, 20 mL; H2. b Yields based on GC analysis. | |||||
| Effect of solvent | |||||
| 1 | Ethanol | 35 | 80 | 8 | 48 |
| 2 | DCM | 35 | 80 | 8 | 83 |
| 3 | ACN | 35 | 80 | 8 | 76 |
| 4 | DMF | 35 | 80 | 8 | 90 |
| 5 | Water | 35 | 80 | 8 | 94 |
| 6 | Toluene | 35 | 80 | 8 | 71 |
| Effect of hydrogen pressure/bar | |||||
| 7 | Water | 25 | 80 | 8 | 27 |
| 8 | Water | 30 | 80 | 8 | 54 |
| 9 | Water | 35 | 80 | 8 | 94 |
| 10 | Water | 40 | 80 | 8 | 94 |
| Effect of temperature | |||||
| 11 | Water | 35 | 40 | 8 | 20 |
| 12 | Water | 35 | 60 | 8 | 54 |
| 13 | Water | 35 | 80 | 8 | 94 |
| 14 | Water | 35 | 100 | 8 | 92 |
| Effect of time | |||||
| 15 | Water | 35 | 80 | 4 | 52 |
| 16 | Water | 35 | 80 | 6 | 73 |
| 17 | Water | 35 | 80 | 8 | 94 |
Next, we studied the effect of the solvent on direct reductive amination and observed that the nature of the solvent affected the conversion of the reaction. In polar solvents like ethanol (48%), DCM (83%), acetonitrile (ACN) (76%), DMF (90%) and water (94%) good to excellent yield of the desired product was obtained (Table 2, entries 1–5). Thereafter a non-polar solvent namely toluene (71%) was screened but lower yield of the desired product was obtained (Table 2, entry 6). It is worth mentioning that the conversion was improved considerably on moving from an organic solvent to water in the presence of PS-Pd–NHC as a catalyst furnishing excellent yield of the desired product. Water being an environmentally benign, safe, and inexpensive solvent was selected for further optimization studies. Further the effect of hydrogen pressure on the reaction outcome was investigated. It was observed that increasing the hydrogen pressure from 25 bar to 40 bar has increased the yield of the desired product where at 35 bar hydrogen pressure 94% yield was obtained while further increase in H2 pressure had no profound effect (Table 2, entries 7–10). In order to examine the effect of temperature on the reaction outcome, reactions were carried out at different temperatures ranging from 40–100 °C (Table 2, entries 11–14). It was observed that at 40 °C the yield of the desired product was low whereas with the increase in temperature up to 100 °C, 92% yield of the desired product was obtained within 8 h (Table 2, entries 11–14). Hence, the final optimized reaction parameters for direct reductive amination were aniline (5 mmol), benzaldehyde (6 mmol), PS-Pd–NHC complex (0.15 mol%), H2 pressure (35 bar), water as solvent (20 mL), temperature (80 °C), and reaction time (8 h).
In order to explore the generality of the developed protocol, we screened several aldehydes and ketones with different aromatic, aliphatic substituted amines and results obtained are summarized in Table 3. The reaction of benzaldehyde with aniline provided the corresponding secondary amine in good yield (Table 3, entry 1). It was observed that aromatic amines containing electron donating or withdrawing groups were well tolerated under present reaction conditions. Aromatic amines with electron donating groups provide excellent yield of the corresponding products (Table 3, entries 2 and 3), whereas aromatic amines having electron withdrawing groups furnish considerable yield of the desired product (Table 3, entries 4 and 5). To our delight aliphatic amines such as benzyl amine, 1-phenylethanamine, cyclohexanamine and butan-1-amine furnished excellent yield of the corresponding product (Table 3, entries 6–9). Furthermore, we screened different secondary amines like morpholine and piperazine exhibiting good yields (Table 3, entries 10 and 11). Cinnamaldehyde also provided appreciable yield with aniline by complete reduction of the double bond (Table 3, entry 12).
| Entry | Aldehyde | Amine | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: aldehyde, 6 mmol; amine, 5 mmol; catalyst, 0.15 mol%; water, 20 mL; H2 pressure, 35 bar; temp., 80 °C; time, 8 h. b Isolated yield. | ||||
| 1 |

|

|
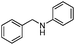
|
93 |
| 2 |
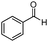
|

|

|
92 |
| 3 |

|
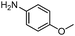
|

|
93 |
| 4 |

|

|
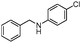
|
73 |
| 5 |

|
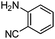
|

|
65 |
| 6 |

|
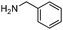
|
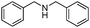
|
91 |
| 7 |

|

|

|
93 |
| 8 |
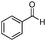
|

|

|
91 |
| 9 |

|

|

|
91 |
| 10 |

|
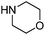
|

|
88 |
| 11 |

|
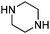
|

|
67(53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47) 47) |
| 12 |
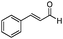
|
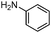
|

|
89 |
| 13 |

|

|

|
73 |
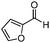
| 14 |
|

|

|
87 |
| 15 |

|

|

|
93 |
| 16 |

|

|

|
73 |
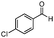
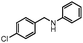
| 17 |
|

|
|
71 |
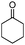
| 18 |
|
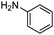
|
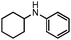
|
91 |
| 19 |
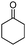
|

|

|
94 |
Further, to extend the scope of the developed protocol, heterocyclic amines like 2-amino pyridine and 2-furaldehyde were screened providing good yield of the desired product (Table 3, entries 13 and 14). Moreover, the applicability of the PS-Pd–NHC catalyst to different electron donating and withdrawing substituted aldehydes with aniline was investigated. 4-Methoxybenzaldehyde furnished 93% yield (Table 3, entry 15), whereas 2-hydroxybenzaldehyde and 4-chlorobenzaldehyde provided 73% and 71% yield respectively (Table 3, entries 16 and 17). Aliphatic ketone like cyclohexanone was also screened for the present protocol. Reaction of cyclohexanone with aniline provided 91% yield of the desired product, while with morpholine furnished excellent yield of the desired product (Table 3, entries 18 and 19). Hence, in general the obtained results highlight the development of a simple protocol using a heterogeneous palladium complex which endows excellent reductive amination of various substituted carbonyl compounds with different amines affording good to excellent yield of the desired products.
To make the synthetic protocol more economical, recyclability study of the polymer bound Pd–NHC complex was examined for direct reductive amination of benzaldehyde with aniline (Fig. 1). We observed that the catalyst was highly active under the present reaction conditions and could be effectively reused for six consecutive recycles. The slight decrease in yield observed for the sixth recycle might be due to handling loss of the catalyst during study. In this context we examined the ICP-AES analysis of filtrates for the 1st and 4th recycle runs and they were found below detectable level (0.01 ppm) of palladium in solution, thus resulting in no significant leaching of palladium.
 | ||
| Fig. 1 Catalyst recyclability study. Reaction conditions: benzaldehyde, 6 mmol; aniline, 5 mmol; catalyst, 0.15 mol%; water, 20 mL; H2 pressure, 35 bar; temperature, 80 °C; time, 8 h. Yield based on GC analysis. | ||
Conclusions
In conclusion, we have developed an efficient, heterogeneous and recyclable catalyst (PS-Pd–NHC) for direct reductive amination of carbonyl compounds with various primary and secondary aromatic, acyclic and cyclic amines, to afford the corresponding secondary and tertiary amines in good to excellent yields. The present protocol employs use of a stable heterogeneous recyclable catalyst, easily available hydrogen gas and water as a solvent, thus adding a credit towards development of greener methodologies for organic synthesis. The complex exhibited remarkable activity in aqueous medium and was effectively recycled up to six consecutive cycles with maintenance of appreciable catalytic activity.Experimental
All chemicals and reagents were purchased from firms of repute with their highest purity available and were used without further purification. The progress of the reaction was monitored by gas chromatograph (Perkin Elmer Clarus 400 GC) equipped with a capillary column (Elite-1, 30 m × 0.25 mm × 0.25 μm) and a flame ionization detector (FID). The product was purified by column chromatography on silica gel (100–200 mesh). All the products are well known in the literature and were confirmed by 1H NMR, 13C NMR, and GC-MS analysis. The polymer-supported palladium–N-heterocyclic carbene complex (PS-Pd–NHC) used was prepared according to the reported procedure in the literature19 and characterized by using different spectroscopic techniques such as solid state 13C NMR and FTIR analysis. Loading of the palladium catalyst on a chloromethyl polystyrene resin support was evaluated by ICP-AES analysis and was found be to about 0.29 mmol g−1 of support.Typical procedure for reductive amination of aldehydes with amines
To a 100 mL stainless steel high pressure reactor were added aldehyde (6 mmol) and amine (5 mmol), resulting in a white opaque solution indicating the formation of an imine intermediate. To this 20 mL solvent (distilled water) was added and finally 25 mg (0.15 mol%) polymer supported Pd–NHC complex was added. The reaction mixture was then pressurized to 35 bar of hydrogen pressure; the reactor was heated to 80 °C and stirred for 8 h at 600 rpm. After completion of reaction, the reactor was cooled to room temperature and the remaining hydrogen was carefully vented and then the reactor was opened. The product was extracted in ethyl acetate (10 mL × 2). The extracts were dried over sodium sulfate and the solvent was evaporated in vacuum to obtain the crude product which was then purified by column chromatography silica gel (100–200 mesh size), with petroleum ether/ethyl acetate (PE–EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05) as eluent to afford a pure product. After phase separation, the aqueous layer containing the suspended PS-Pd–NHC complex was recovered by simple filtration and employed for the next run. The reaction mixture was analyzed by GC and the products were confirmed by GCMS and 1H NMR.
05) as eluent to afford a pure product. After phase separation, the aqueous layer containing the suspended PS-Pd–NHC complex was recovered by simple filtration and employed for the next run. The reaction mixture was analyzed by GC and the products were confirmed by GCMS and 1H NMR.
Experimental procedure for recycling of the PS-Pd–NHC catalyst for direct reductive amination of benzaldehyde with aniline
The reaction was carried out as mentioned above in a typical experimental procedure. However after completion of reaction, the reactor was cooled to room temperature and the remaining hydrogen gas was carefully vented and the reactor opened, the product was extracted in ethyl acetate and the aqueous layer containing the suspended catalyst was filtered, the filtered catalyst was washed vigorously with distilled water (5 × 10 mL) and methanol (5 × 10 mL) to remove all traces of the product or reactant present. The filtered catalyst was then dried under reduced pressure and kept for activation at 80 °C for a period of 4 h prior to the next recycle. The dried catalyst was then used for a catalyst recyclability experiment and it was observed that the recovered catalyst could be reused up to six consecutive cycles affording good to appreciable yield of the desired product.Characterization data of some selected compounds
Acknowledgements
The authors (DBB) express their gratitude towards UGC-SAP (University Grant Commission, India) for providing financial assistance.Notes and references
- T. C. Nugent and M. El-Shazly, Adv. Synth. Catal., 2010, 352, 753 Search PubMed; S. Gomez, J. A. Peters and T. Maschmeyer, Adv. Synth. Catal., 2002, 344, 1037 CrossRef CAS; E. M. Gordon, R. W. Barret, W. J. Dower, S. P. A Fodor and M. A. Gallop, J. Med. Chem., 1994, 37, 1385 CrossRef; B. Merla and N. Risch, Synthesis, 2002, 1365 Search PubMed.
- D. B. Sharp, Herbicides Chemistry, Degradation and mode of action, 1988 Search PubMed; A. Ricci, Modern amination method, Wiley, New York, 2000 Search PubMed.
- E. E. Boros, J. B. Thompson, S. R. Katamreddy and A. J. Carpenter, J. Org. Chem., 2009, 74, 3587 CrossRef CAS; S. C. A. Sousa and A. C. Fernandes, Adv. Synth. Catal., 2010, 352, 2218 CrossRef.
- A. Kumar, S. Sharma and R. A. Maurya, Adv. Synth. Catal., 2010, 352, 2227 CrossRef CAS; C. Wang, A. Pettman, J. Basca and J. L. Xiao, Angew. Chem., Int. Ed., 2010, 49, 7548 CrossRef.
- A. Pelter, R. M. Rosser and S. Mills, J. Chem. Soc., Perkin Trans. 1, 1984, 717 RSC; M. D. Bomann, I. C. Guch and M. DiMare, J. Org. Chem., 1995, 60, 5995 CrossRef CAS.
- J. R. Brussee, A. T. M. van Benthem, C. G. Kruse and A. van der Gen, Tetrahedron: Asymmetry, 1990, 1, 163 CrossRef CAS.
- S. Bhattacharyya, A. Chatterjee and S. K. Duttachowdhury, J. Chem. Soc., Perkin Trans. 1, 1994, 1 RSC.
- A. F. Abdel-Magid, C. A. Maryanoff and K. G. Carson, Tetrahedron Lett., 1990, 31, 5595 CrossRef CAS; A. F. Abdel-Magid, K. G. Carson, B. D. Harris, C. A. Maryanoff and R. D. Shah, J. Org. Chem., 1996, 61, 3849 CrossRef; H. O. Kim, B. Carrol and M. S. Lee, Synth. Commun., 1997, 27, 2505 CrossRef; V. A. Tarasevich and N. G. Kozlov, Russ. Chem. Rev., 1999, 68, 55 CrossRef.
- B. C. Ranu and A. Sarkar, J. Org. Chem., 1998, 63, 370 CrossRef CAS.
- I. Shibata, T. Suwa, E. Sugiyama and A. Baba, Synlett, 1998, 1081 CrossRef CAS; I. Shibata, T. Moriuchi-Kawakami, D. Tanizawa, T. Suwa, E. Sugiyama, H. Matsuda and A. Baba, J. Org. Chem., 1998, 63, 383 CrossRef.
- R. F. Borch, M. D. Bernstein and H. D. Durst, J. Am. Chem. Soc., 1971, 93, 2897 CrossRef CAS; R. F. Borch and A. I. Hassid, J. Org. Chem., 1972, 37, 1673 CrossRef; P. Marchini, G. Liso, A. Reho, F. Liberatone and F. M. Moracci, J. Org. Chem., 1975, 40, 3453 CrossRef; C. F. Lane, Synthesis, 1975, 135 CrossRef.
- O.-Y. Lee, K.-L. Law and D. Yang, Org. Lett., 2009, 11, 3302 CrossRef CAS.
- H. K. Ikuya Shibata, Y. Yasaka, S. Tsunoi, M. Yasudaa and A. Baba, Chem. Commun., 2006, 4189 Search PubMed; P. Dien Pham, P. Bertus and S. Legoupy, Chem. Commun., 2009, 6207 RSC; R. Apodaca and W. Xiao, Org. Lett., 2001, 3, 1745 CrossRef CAS; S. Enthaler, Catal. Lett., 2011, 141, 55 CrossRef; S. Enthaler, ChemCatChem, 2010, 2, 1411 CrossRef.
- M. Zhang, H. Yang, Y. Zhang, C. Zhu, W. Li, Y. Cheng and H. Hu, Chem. Commun., 2011, 47, 6605 RSC; C. Wang, A. Pettman, J. Bacsa and J. Xiao, Angew. Chem., Int. Ed., 2010, 49, 7548 CrossRef CAS.
- L. Xing, C. Cheng, R. Zhu, B. Zhang, X. Wang and Y. Hu, Tetrahedron, 2008, 64, 11783 CrossRef CAS; M. M. Dell'Anna, P. Mastrorilli, A. Rizzuti and C. Leonelli, Appl. Catal., A, 2011, 401, 134 CrossRef.
- Organic reactions in water, ed. U. M. Lindstrom, Blackwell publishing, Oxford, 2007 CrossRef CAS; U. M. Lindstrom, Chem. Rev., 2002, 102, 2751 CrossRef CAS; R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef; Y. Wang, J. Yao, H. Li, D. Su and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 2362 CrossRef.
- A. Libineau, J. Auge and Y. Queneau, Synthesis, 1994, 741 CrossRef CAS; X. Li, L. Li, Y. Tang, L. Zhong, L. Cun, J. Zhu, J. Liao and J. Deng, J. Org. Chem., 2010, 75, 2981 CrossRef; M. M. Wang, L. He, Y. M. Liu, H. Y. He and K. N. Fan, Green Chem., 2011, 13, 602 RSC.
- J. W. Sprengers, J. Wassenaar, N. D. Clement, K. J. Cavell and C. J Elsevier, Angew. Chem., Int. Ed., 2005, 44, 2026 CrossRef CAS; H. J. Kim, M. Kim and S. Chang, Org. Lett., 2011, 13, 2368 CrossRef; P. Hauwert, R. Boerleider, S. Warsink, J. J. Weigand and C. J. Elsevier, J. Am. Chem. Soc., 2010, 132, 16900 CrossRef; N. Patil, Angew. Chem., Int. Ed., 2011, 50, 1759 CrossRef; J. H. Park, S. V. Bhilare and S. W. Youn, Org. Lett., 2011, 13, 2228 CrossRef; T. Droge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940 CrossRef.
- J.-W. Byun and Y.-S. Lee, Tetrahedron Lett., 2004, 45, 1837 CrossRef CAS; Z. S. Qureshi, K. M. Deshmukh, P. J. Tambade and B. M. Bhanage, Synthesis, 2011, 243 Search PubMed; J. Kim, B. Jun, J. Byun and Y. Lee, Tetrahedron Lett., 2004, 45, 5827 CrossRef; W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290 CrossRef; L. Li, J. Wang, C. Zhou, R. Wang and M. Hong, Green Chem., 2011, 13, 2071 RSC; B. S. Yong and S. P. Nolan, Chemtracts, 2003, 16, 205 Search PubMed; K. J. Cavell and D. S. McGuinness, Coord. Chem. Rev., 2004, 248, 671 CrossRef; K. V. S. Ranganath, J. Kloesges, A. H. Schafer and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 7786 CrossRef.
- D. B. Bagal, Z. S. Qureshi, K. P. Dhake, S. R. Khan and B. M. Bhanage, Green Chem., 2011, 13, 1490 RSC.
- M. D. Bhor, M. J. Bhanushali, N. S. Nandurkar and B. M. Bhanage, Tetrahedron Lett., 2008, 49, 965 CrossRef CAS; M. J. Bhanushali, N. S. Nandurkar, M. D. Bhor and B. M. Bhanage, Tetrahedron Lett., 2007, 48, 1273 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and catalyst characterization data and 1H NMR and 13C NMR spectra of selected products. See DOI: 10.1039/c1cy00392e |
| This journal is © The Royal Society of Chemistry 2012 |


