Reducibility and toluene hydrogenation activity of nickel catalysts supported on γ-Al2O3 and κ-Al2O3
Jinsoon
Choi
,
Shihua
Zhang
and
Josephine M.
Hill
*
Department of Chemical and Petroleum Engineering, Schulich School of Engineering, University of Calgary, 2500 University Dr NW, Calgary, AB T2N 1N4, Canada. E-mail: jhill@ucalgary.ca; Fax: +1 403 284 4852; Tel: +1 403 210 9488
First published on 17th October 2011
Abstract
Metastable κ-Al2O3 was produced by thermal treatment of γ-Al2O3 and then Ni catalysts were prepared on both κ-Al2O3 and γ-Al2O3. Catalysts containing both low (5 wt.%) and high (20 wt.%) amounts of Ni were tested for activity towards toluene hydrogenation and characterized with X-ray diffraction, temperature-programmed reduction, and transmission electron microscopy. In order to better understand the behaviours of these catalysts, total reducibility, surface oxide reducibility, metal dispersion, and number of adsorption sites were compared with catalytic activity. Of these factors, only surface oxide reducibility was directly correlated with activity – the more easily the surface oxide was reduced the more active the catalyst was. Catalytic activity increased in the following order: Ni5/γ-Al2O3<Ni5/κ-Al2O3<Ni20/κ-Al2O3<Ni20/γ-Al2O3. The number of metallic active sites is usually considered as a major factor to determine the corresponding activity, but in this study reducibility changes in surface Ni sites were more influential to the activity than the number of active sites.
1. Introduction
Alumina is one of the most commonly used catalyst support materials because of its intrinsic properties such as high thermal stability, high mechanical strength, large surface area and mild acidity.1,2 There remains, however, a limited understanding of how the properties of the various crystalline phases of alumina, including γ-Al2O3, κ-Al2O3, θ-Al2O3, and α-Al2O3, relate to the performance of catalysts prepared with these aluminas. The physical/chemical properties, such as packing density, surface energy, band gap, valence bandwidth and cation site vacancies, vary on aluminas as the crystal structure deforms through thermal treatment from γ-Al2O3 to intermediates and finally to α-Al2O3.3–5 The Al2O3 ceramic system is known to exist in a number of metastable polymorphic forms, such as γ (cubic spinel), δ (tetragonal), θ (monoclinic), η (cubic spinel), κ (orthorhombic), χ (cubic), and β (hexagonal).6 The pathway of the phase transition of alumina depends on the precursor; for example, gibbsite → χ-Al2O3 (300–500 °C) → κ-Al2O3 (800–1150 °C) → α-Al2O3 (1150 °C-), boehmite → γ-Al2O3 (500–850 °C) → δ-Al2O3 (850–1050 °C) → θ-Al2O3 (1050–1150 °C) → α-Al2O3 (1150 °C-), and bayerite → η-Al2O3 (300–500 °C) → θ-Al2O3 (880–1150 °C) → α-Al2O3 (1150 °C-).7 The specific structure (i.e., crystalline deformation) of alumina can significantly impact the catalytic activity.8,9The reducibility of metal sites has been suggested as a key factor to determine catalytic activity10,11 because reducibility is an indication of the metal stability in the reduced state when reaction occurs through a catalytic red-ox cycle such as oxidative addition of reactants and reductive elimination of products. Often studies have focused on the properties of the bulk metal oxides, however, instead of surface metallic sites where the actual red-ox reaction takes place.
A chemical reaction over a catalyst requires an active site to be stable at both oxidized and reduced zero-valent states when the reaction takes place only on the active site. An active site that is unstable at a zero-valent state makes the catalyst susceptible to poisoning by oxidation. When the metal becomes oxidized, dissociative addition of reactants to the active site, i.e. weak adsorption ability, is prohibited. The ability of nickel sites to be oxidized depends on the interaction between the metal and the support. This interaction depends on the type of metal, the type of support, and the metal particle size. The reduction process itself has been previously described mainly by one of two models.12,13 The first model is the nucleation model in which clusters of atoms aggregate into a new phase; while the second model involves a continuous reduction through phase transition of the oxide interface and the rate of oxide reduction is proportional to the size of the particles. The direct relationship between reducibility and catalytic activity still remains unclear.
In this study, Ni catalysts supported on two different aluminas (γ-Al2O3 and κ-Al2O3) have been prepared, characterized and tested in order to try to elucidate a relationship between surface reducibility and catalytic behaviour. Two different metal loadings (5 wt.% and 20 wt.%) were used so that the effect of nickel particle size, as well as the type of support, could be evaluated. The catalytic activity of the prepared catalysts was determined for toluene hydrogenation at 1.38 MPa and temperatures between 120 °C and 290 °C, and the catalysts were characterized using temperature-programmed reduction, X-ray diffraction, and scanning transmission electron microscopy.
2. Experimental
2.1. Catalyst preparation
γ-Al2O3 (activated, neutral, −60 mesh powder, Alfa Aesar, Ward Hill, MA, USA) was thermally treated in air at 1000 °C for 3 h to produce κ-Al2O3. Wet impregnation of the γ-Al2O3 and κ-Al2O3 aluminas with nickel nitrate hexahydrate (Ni(NO3)2·6H2O, 99.4%, J.T. Baker, Phillipsburg, NJ, USA) was performed to prepare catalysts with target loadings of 5 wt.% and 20 wt.% Ni. After calcination at 500 °C, 600 °C, or 700 °C in air, the catalysts were treated with ex situreduction at 450 °C–750 °C for 2 h followed by a passivation step using 4.98% O2 in He at room temperature for 2 h. Reduction temperatures were determined based on the TPR profiles (Fig. 3), and chosen as a balance between maximizing reduction and minimizing sintering. As will be discussed in the Results, reduction temperatures between 450 °C and 500 °C will result in similar catalysts in terms of characteristics and activities. The alumina-supported Ni catalysts were named according to Ni content, type of alumina, and temperatures of calcination and ex situreduction. For example, Ni20/κ-Al2O3 (500–450) refers to 20 wt.% Ni supported on κ-Al2O3 with calcination at 500 °C and ex situreduction at 450 °C, while Ni5/γ-Al2O3 (500-x) refers to a 5 wt.% Ni catalyst supported on γ-Al2O3 with calcination at 500 °C and no reduction.2.2. Characterization
Temperature-programmed reduction (TPR) was conducted on a ChemBET instrument (Quantachrome, Boynton Beach, FL, USA) to determine both qualitative and quantitative changes in oxygen species within Ni oxides before and after ex situreduction. X-ray diffraction (XRD) powder patterns were obtained on a Multiflex X-ray diffractometer (Rigaku, Woodlands, TX, USA) using Cu/Kα radiation (λ = 1.54056 Å) at 40 kV tube voltage and 20 mA tube current with a scan rate of 2 degree/min. Surface area and pore volume were measured by nitrogen physisorption at −196 °C with a TriStar instrument (Micromeritics, Norcross, GA, USA). All samples were degassed with flowing nitrogen at 250 °C for 4 h prior to physisorption. Transmission electron microscope (TEM) images were obtained with a Tecnai F20 (FEI, Hillsboro, OR, USA) instrument using scanning mode (STEM) in order to observe nickel particles and elemental analysis was performed with an energy dispersive X-ray (EDX) system attached to the microscope. The samples were mounted on carbon-coated copper grids.2.3. Activity measurement
Toluene hydrogenation was performed in a plug-flow reactor with 0.1 g of catalyst at a reaction pressure of 1.38 MPa and a liquid hourly space velocity (LHSV) of 2.4 h−1. The catalysts were reduced in situ for 2 h at 290 °C before the reaction was started. The catalytic activities were measured at temperatures between 120 °C and 290 °C in several steps. Initially the activities of the catalysts were measured at 290 °C for 20 h to ensure that steady state had been reached. After this time, the furnace was switched off and the activities were measured every 15 min as the reactor cooled to room temperature. The molar ratio of H2/toluene/N2 in the feed to the reactor was 90/1/43.5. The gas lines were heated to 180 °C in order to avoid condensation of toluene. The effluent was analyzed by a gas chromatograph (GC 6890, Agilent, Santa Clara, CA, USA), equipped with a flame ionization detector employing a capillary column (60 m × 0.32 mm, J&W GS-GasPro), to determine the conversion of toluene and the selectivity to methylcyclohexane.3. Results and discussion
Nickel catalysts of 5 wt.% and 20 wt.% were prepared with supports of γ-Al2O3 and κ-Al2O3 and tested for toluene hydrogenation. Fig. 1 shows the hydrogenation activity of the catalysts—Ni5/γ-Al2O3 (500–500), Ni5/κ-Al2O3 (500–500), Ni20/γ-Al2O3 (500–450), and Ni20/κ-Al2O3 (500–450)—as a function of reaction temperature. For the 5 wt.% catalysts, the conversion of toluene was much lower over the catalyst supported on γ-Al2O3 than on κ-Al2O3. For example, at 290 °C the conversions were 28% and 98%, respectively. The activities of both catalysts declined to essentially zero at 120 °C. This low activity was not likely from deactivation because the activity could be recovered by increasing the temperature. Instead the low activity is likely a consequence of kinetic limitations at the lower temperatures. Increasing the Ni content from 5 wt.% to 20 wt.% reduced the difference in the activities of the catalysts supported on different phases of alumina. In this case, the catalyst supported on γ-Al2O3 was more active over all reaction temperatures tested. The selectivities to methylcyclohexane (MCH) were over 98.5% for all of the Ni catalysts. There was, however, an activity decrease after 215 °C for Ni20/κ-Al2O3. It is typical for catalysts to have an optimum in activity with temperature. The activities of the Ni20/γ-Al2O3 and Ni5/κ-Al2O3 catalysts also decrease above 200 °C. This optimum occurs for a variety of reasons but may be related to decreased adsorption of the reactants at higher temperatures.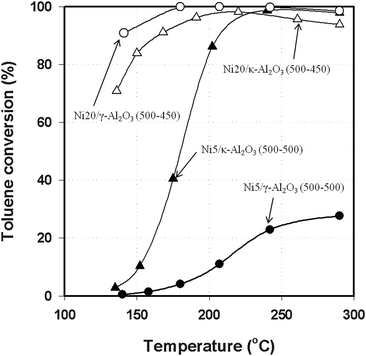 | ||
| Fig. 1 Toluene conversion over Ni catalysts as a function of reaction temperature at 1.38 MPa with a LHSV of 2.4 h−1. | ||
Physical characteristics of alumina supports and Ni catalysts are shown in Table 1. Note, that two different batches of gamma-alumina were used for the Ni20 and Ni5 catalysts because the first bottle of alumina was used up after the preparation of the Ni20 catalysts. Although, the batches of alumina should be the same, the physical properties are slightly different. For example, the surface area varies between 96 m2 g−1 and 160 m2 g−1.
Further characterization of these catalysts was done to better understand the difference in activities. First, the catalyst supports were examined with XRD as shown in Fig. 2. The spectrum for each support was compared to the standard spectra for κ-Al2O3 (JCPDS 04-0878) and γ-Al2O3 (JCPDS 10-0425). There is good agreement between the XRD spectra of the supports and the reference materials. The κ-Al2O3 was derived from the γ-Al2O3via thermal treatment, and is a complex ionic crystal with low structural symmetry, a higher band gap than γ-Al2O3, and hexagonal close packing compared to face-centered cubic packing for γ-Al2O3.3 The structure of κ-Al2O3 is orthorhombic with oxygen ions in close-packed ABAC stacking and aluminum ions occupying both tetrahedral (1/4) and octahedral (3/4) interstitial sites.4γ-Al2O3 is a defective spinel phase of alumina with cation site vacancies (on octahedral sites) randomly distributed throughout the crystal, with Al ions on 43% of the tetrahedral sites, a smaller bad gap, and a wider valence bandwidth.5 Ruberto claimed that the band gap consistently decreased with increasing fraction of tetrahedrally coordinated Al in the structure, and that these tetrahedral Al ions have a higher charge localization than the Al ions on octahedral sites.3 Although no theoretical calculations on metal-support interactions have been conducted in this study, the presence of Al ions with higher charge localization in the γ-Al2O3 structure is likely to produce acid sites and consequently result in stronger oxygen bonds between the metal and the support.
 | ||
| Fig. 2 XRD patterns of pure γ-Al2O3, and κ-Al2O3 derived from the γ-Al2O3 by thermal treatment at 1000 °C for 3 h. Reference peaks for γ-Al2O3 and κ-Al2O3 were from JCPDS 10-0425 and 04-0878, respectively. | ||
The differences in oxygen bond strength formed on or in oxidized catalysts can be probed through temperature-programmed reduction (TPR) as shown in Fig. 3. A bulk Ni(II)O (99%, Alfa Aesar, Ward Hill, MA, USA) was also characterized for comparison. The peak areas from the TPR profiles represent the hydrogen consumption by the total amount of Ni oxide in the catalyst. For the determination of peak area, baselines were chosen assuming that H2 consumption above 900 °C was negligible. Various baseline corrections resulted in less than ±5% difference in the hydrogen consumption. The average consumptions of hydrogen for 5 wt.% Ni and 20 wt.% Ni catalysts were 0.85 mmol g-cat−1 and 3.31 mmol g-cat−1, respectively, while the theoretical values are 0.84 mmol g-cat−1 and 3.23 mmol g-cat−1. Thus, the actual metal loadings are close to the target loadings.
 | ||
| Fig. 3 TPR profiles of Ni catalysts after calcination: a. Ni5/γ-Al2O3 (500-x), Ni5/κ-Al2O3 (500-x), and b. Ni20/γ-Al2O3 (500-x), Ni20/κ-Al2O3 (500-x) and pure NiO. | ||
The reducibility of the Ni oxide, and hence oxygen bond strength, in each catalyst corresponded to the types of alumina and the Ni content. For the 5 wt.% Ni catalysts (Fig. 3a), Ni supported on γ-Al2O3 was reduced between 380 °C and 900 °C, while the Ni on κ-Al2O3 was reduced between 325 °C and 800 °C, indicating that the interaction between Ni and the support was different depending on the structure of alumina. In contrast, bulk NiO (Fig. 3b) was reduced at lower temperatures, between 280 °C and 445 °C, than the supported metals as observed previously.14 When the Ni content was increased to 20 wt.%, the reduction behavior changed. The TPR profile for Ni20/γ-Al2O3 (500-x) (Fig. 3b) had two main peaks at temperatures between 280 °C and 435 °C, and 435 °C to 900 °C. The Ni20/κ-Al2O3 (500-x) sample was easier to reduce with peak temperatures between 280 °C and 610 °C. The profiles of both 20 wt.% catalysts contained a low temperature peak similar to that seen for bulk NiO.
TEM images of Ni5/γ-Al2O3 (500–500), Ni5/κ-Al2O3 (500–500), Ni20/γ-Al2O3 (500–450), and Ni20/κ-Al2O3 (500–450) are shown in Fig. 4. The distribution of nickel particles varied with the types of support as well as nickel content. No nickel particles were evident on Ni5/γ-Al2O3 (500–500) while 5–15 nm particles were evident on Ni5/κ-Al2O3 (500–500). Increasing the nickel content from 5 wt.% to 20 wt.% nickel resulted in significantly larger particles, namely 10–120 nm on Ni20/γ-Al2O3 (500–450) and 10–70 nm on Ni20/κ-Al2O3 (500–450). These large nickel particles were well distributed on the alumina surfaces. At lower Ni contents, the dispersion was higher on the γ-Al2O3-supported catalyst but at higher Ni contents the dispersion was higher on the κ-Al2O3-supported sample. The specific surface areas of the aluminas (γ-Al2O3, 166.7 m2 g−1; κ-Al2O3, 29.6 m2 g−1) had no relation with the dispersion or particle sizes of Ni.
 | ||
| Fig. 4 STEM images of Ni5/γ-Al2O3 (500–500), Ni5/κ-Al2O3 (500–500), Ni20/γ-Al2O3 (500–450) and Ni20/κ-Al2O3 (500–450). The arrows point to Ni particles. | ||
Fig. 5 shows the reduction patterns of passivated catalysts. The hydrogen uptake is related to reduction of either surface oxygen formed from the passivation process or lattice oxygen from Ni oxides that were not completely reduced during the reduction process. The lower temperature peaks (140 °C–320 °C) in Fig. 5 are related to the former (i.e., reduction of surface oxides). With 5 wt.% Ni, the low temperature peak is smaller for the γ-Al2O3-supported catalyst and the peak maximum occurs at a higher temperature (280 °C versus 242 °C). With 20 wt.% Ni, the peak intensity is lower for the γ-Al2O3-supported catalyst but the peak maximum occurs at a lower temperature than for Ni20/κ-Al2O3 (500–450) (190 °C compared to 218 °C). On the γ-Al2O3-supported catalysts, there is significant reduction above 500 °C. The species being reduced at these temperatures likely correspond to the species producing the higher temperature peaks in the TPR of the calcined catalysts (Fig. 3). That is, these species are Ni oxides that were not fully reduced during the reduction process at 500 °C because of strong metal-support bonding. The Ni associated with these Ni oxide species will not be involved in reactions conducted at and below 290 °C.
 | ||
| Fig. 5 TPR profiles of passivated catalysts: a. Ni5/γ-Al2O3 (500–500), Ni5/κ-Al2O3 (500–500), and b. Ni20/γ-Al2O3 (500–450) and Ni20/κ-Al2O3 (500–450). | ||
The XRD patterns of the calcined and reduced catalysts are shown in Fig. 6 with the JCPDS references for NiO and Ni. The catalysts containing 5 wt.% Ni did not have prominent peaks for either Ni oxide or Ni metal after either calcination or reduction, even though particles were visible in the TEM images (Fig. 4) of Ni5/κ-Al2O3(500–500). In contrast, the catalysts containing 20 wt.% Ni on γ-Al2O3 and κ-Al2O3 and calcined at 500 °C had peaks related to NiO with particle sizes of 25.6 nm and 27.0 nm, respectively, while the catalysts reduced at 450 °C had peaks related to metallic Ni with particle sizes of 32.1 nm and 23.2 nm, respectively.
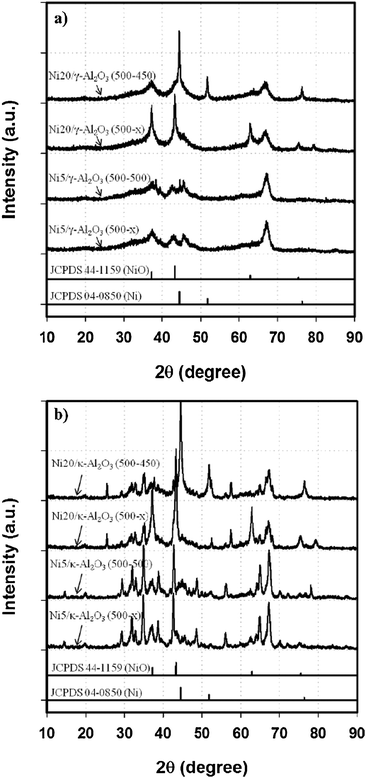 | ||
| Fig. 6 XRD patterns of calcined and reduced catalysts supported on a. γ-Al2O3, and b. κ-Al2O3. Reference peaks for NiO and Ni were from JCPDS 44-1159 and 04-0850, respectively. | ||
To further probe what influences the reducibility of the supported Ni particles, catalysts containing 20 wt.% Ni, which have larger particle sizes (Fig. 4) and thus, more bulk Ni properties, were further characterized with XRD and TPR. Catalysts supported on γ-Al2O3 were reduced at various temperatures, while catalysts supported on κ-Al2O3 were calcined at various temperatures, and then reduced and passivated.
The XRD analysis (data not shown) of Ni20/γ-Al2O3 indicated that an increase in reduction temperature up to 750 °C had an insignificant effect on particle size growth. In this study, the Ni particle sizes on Ni20/γ-Al2O3 as determined by XRD using the Scherrer equation with 44.4° 2 theta (Ni (111) face) were 28.3 nm, 32.1 nm, 31.8 nm, 31.7 nm, 29.1 nm, 28.1 nm and 29.6 nm for reduction at 350 °C, 450 °C, 500 °C, 550 °C, 600 °C, 650 °C and 750 °C, respectively. Given the inaccuracies of the application of the Scherrer, these particle sizes are all within the error of the measurements and calculations. Thus, there was no relationship between reduction temperature and particle size in this reduction temperature range.
Fig. 7 shows TPR profiles of Ni20/γ-Al2O3 catalysts reduced ex situ at various temperatures. Peaks occurring at temperatures below 300 °C correspond to surface oxygen while the higher temperature peaks correspond to bulk oxygen (i.e., lattice oxygen of Ni oxides). As the reduction temperature increased, the relative amount of surface to bulk oxygen increased. All catalysts were passivated after reduction. The TPR profile for the catalyst reduced ex situ at 750 °C (Ni20/γ-Al2O3 (500–750)) is consistent with complete reduction of the Ni and formation of only a surface oxide during passivation. XRD results (data not shown) indicate that the amount of metallic Ni increased 2.4 times as the reduction temperature increased from 350 °C to 750 °C.
 | ||
| Fig. 7 TPR profiles of Ni20/γ-Al2O3 catalysts reduced at different temperatures. | ||
The amount of surface oxygen and onset temperature are essentially the same for reduction at 550 °C, 600 °C and 750 °C consistent with a constant particle size after reduction at these temperatures as estimated by XRD. At lower ex situreduction temperatures, there is less surface oxygen, the onset temperature has increased, and there is significantly more bulk oxide present. These results suggest that the fraction of Ni oxides in the core of the particle influences reducibility. In the XRD spectra (data not shown) of these catalysts, peaks corresponding to NiO are not present after reduction at 500 °C. As there is a significant bulk oxide peak in the TPR profile for the Ni20/γ-Al2O3 (500–500) catalyst (Fig. 7), these Ni oxides are likely present in an amorphous form rather than in the form of Ni(II)O crystalline, which would be visible in the XRD spectra.
The calcination process, during which organic precursors are removed, oxidizes the metal species and generates oxygen bonds between these metal species and the oxide support. The higher the calcination temperature, the stronger the oxygen bonds created, and occasionally the bigger the particles formed. Fig. 8a shows TPR profiles of 20 wt.% Ni catalysts supported on κ-Al2O3 calcinced at different temperatures. As the calcination temperature increased, the reduction temperatures shifted to higher temperatures. For example, the peak intensity at 390 °C decreased while the peak intensity at 775 °C increased. These shifts indicate that the catalysts became less reducible and created stronger Ni–O bonds as the treatment temperature increased. Crystalline NiAlO4, which can form at 700 °C in the presence of steam15 or thermal treatment above 1100 °C,9 was not observed in the XRD patterns (data not shown). The relative amounts of hydrogen consumed for catalysts calcined at 500 °C, 600 °C, and 700 °C were 1, 1.05, and 1.06, respectively, while the NiO particle sizes, as calculated from the XRD profiles, were 27.0 nm, 28.0 nm, and 29.8 nm, respectively. These catalysts were from the same batch and so should all have the same Ni content. Thus, the 6% increase in hydrogen consumption might be related to the degree of Ni oxidized as the treatment temperature increased from 500 °C to 700 °C.
 | ||
| Fig. 8 TPR profiles of Ni20/κ-Al2O3 catalysts after (a) calcination, and (b) reduction at different temperatures. | ||
After reduction and passivation, the TPR profiles of the Ni20/κ-Al2O3 catalysts were much more similar (Fig. 8b) than immediately after calcination (Fig. 8a). These catalysts were reduced at 500 °C for 2 h, and this procedure resulted in the reduction of most of the Ni oxides on the catalysts calcined at 500 °C and 600 °C. The TPR profile of the catalyst calcined at 700 °C (Ni20/κ-Al2O3 (700–500)) contains a peak at 775 °C, indicating that this sample was not fully reduced. Nevertheless, this peak is significantly smaller than that for the catalysts supported on γ-Al2O3, calcined at 500 °C and reduced at temperatures up to 500 °C (Fig. 7). High temperature calcination can lead to strong Ni-oxygen bonds, but mostly κ-Al2O3 exhibited a weaker affinity for Ni and consequently the Ni oxides were more easily reduced. The impact of increased reducibility of the surface oxides can be seen in Fig. 9, which shows that the toluene hydrogenation over Ni20/κ-Al2O3 (500–450) was higher than over Ni20/κ-Al2O3 (700–500). The relative amounts of hydrogen consumed in the TPR were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.923 for the catalysts calcined at 500 °C and 700 °C. Thus, the decrease in number of surface sites would not fully account for the decrease in activity.
0.923 for the catalysts calcined at 500 °C and 700 °C. Thus, the decrease in number of surface sites would not fully account for the decrease in activity.
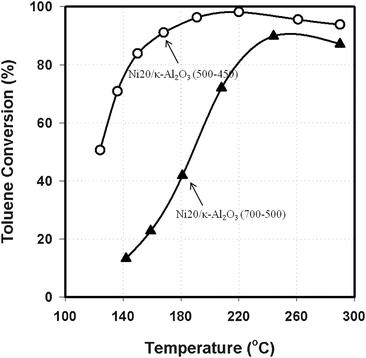 | ||
| Fig. 9 Toluene conversion over Ni20/κ-Al2O3 (500–450) and Ni20/κ-Al2O3 (700–500) as a function of reaction temperature at 1.38 MPa with a LHSV of 2.4 h−1. | ||
The purpose of this study was to determine if there is a relationship between reducibility and activity of Ni catalysts supported on two different forms of alumina at two different metal loadings. Several factors influence reducibility of metal particles including particle size,16 metal content,17 interaction,18 support acidity,19oxidation state,20 and composition (i.e., separate phases versus composite),21 which in turn are influenced by the calcination and reduction conditions. TPR profiles reflect the reducibility of a material or in other words, the metal-oxygen bond strength. It is the surface atoms of a particle that are accessible for reactions and the proportion of surface atoms decreases with particle size as illustrated in Fig. 10. This figure includes a schematic of a particle with a diameter of 1.94 nm and containing 201 Ni atoms.22 The surface energy created by non-saturated fold atoms in corner, edge, and plane sites becomes less significant with increasing Ni particle size.23
 | ||
| Fig. 10 Fraction of surface sites and their type (corner, edge, or face (plane)) as a function of particle size. | ||
The Ni particle sizes on the catalysts used in this study have been observed by TEM and estimated using the TPR profiles and XRD spectra. In the TEM images (Fig. 4), the particle sizes ranged from not detectable to 5–15 nm to 10–70 nm to 10–120 nm on Ni5/γ-Al2O3 (500–500), Ni5/κ-Al2O3 (500–500), Ni20/κ-Al2O3 (500–450), and Ni20/γ-Al2O3 (500–450), respectively. From the TPR profiles (Fig. 5) with the assumption of a single layer of oxygen deposited on the reduced Ni surface (Fig. 10), the particle sizes were 8.1 nm for Ni5/γ-Al2O3, 5.2 nm for Ni5/κ-Al2O3, 12.3 nm for Ni20/κ-Al2O3, and 16.0 nm for Ni20/γ-Al2O3. Finally, from the XRD spectra, the particle sizes were not detectable for the 5 wt.% catalysts, and were 23.2 nm and 32.1 nm for Ni20/κ-Al2O3 (500–450), and Ni20/γ-Al2O3 (500–450), respectively.
The particle sizes are consistent in terms of metal content—typically higher metal loadings are less well dispersed—but contrary to the belief that the stronger the interaction the smaller the particle size. That is, the Ni particles on κ-Al2O3 were smaller than those on the γ-Al2O3. As mentioned, the γ-Al2O3 likely contains acidic sites that will interact with the Ni more strongly than the neutral κ-Al2O3. This strong interaction was seen in the TPR profiles. The bulk (Fig. 3) oxides were reduced at a lower temperature on the κ-Al2O3-supported catalysts, and at higher temperatures on the γ-Al2O3-supported catalysts. The reducibility of the surface oxides, however, depended on the Ni content. At 5 wt.%, the surface oxides were more easily reduced on Ni5/κ-Al2O3 (500–500) than Ni5/γ-Al2O3 (500–500), while at 20 wt.%, the surface oxides were more easily reduced on Ni20/γ-Al2O3 (500–450) than Ni20/κ-Al2O3 (500–450) (Fig. 5). Overall, as the onset temperature of reduction decreased the catalysts became more active in the following order: Ni5/γ-Al2O3 < Ni5/κ-Al2O3 < Ni20/κ-Al2O3 < Ni20/γ-Al2O3. We have observed this relationship between onset temperature of reduction and activity with nickel catalysts supported on other materials including zeolite 13X, silica, alpha alumina, and carbon.
The amount of surface oxygen (i.e., number of active sites, peak areas of lower temperature peaks in Fig. 5), however, did not provide a direct measure of the catalytic activity for toluene hydrogenation, suggesting that on a per site basis, the active sites on Ni20/γ-Al2O3 are considerably more active than those on Ni20/κ-Al2O3. There may be insufficient active sites on Ni5/γ-Al2O3 (500–500) to convert all of the toluene.
The reducibility of surface Ni sites was also influenced by oxygen within the structure. Ni particles containing more bulk oxide were more difficult to reduce. A lower calcination temperature (500 °C versus 700 °C, Fig. 8b) or a higher reduction temperature (750 °C, Fig. 7) reduced the amount of bulk oxide and the temperature at which the surface oxides were reduced.
As the Ni particle size increases the fraction of atoms exposed on the surface decreases (Fig. 10). For example, the fraction of surface atoms decreases from 23.5% (plane 19.7%, edge 3.45%, corner 0.38%) for a particle with a diameter of 5.46 nm (6266 Ni atoms) to 10.4% for a particle with a diameter of 12.5 nm. The Ni particle sizes, calculated from the XRD spectra for the catalysts containing 20 wt.% Ni, were between 28.1–32.1 nm, which corresponds to a fraction of surface atoms of 4.7–4.1%, and so the metallic Ni peak seen in XRD corresponds to the reduction of bulk Ni. Given the higher activity of the catalysts containing 20 wt.% Ni, the fraction of surface sites cannot be the only factor in determining activity but the reducibility of the surface oxides appears to be an important factor.
4. Conclusions
In this study, Ni catalysts supported on two forms of alumina, γ-Al2O3 and κ-Al2O3, with two different metal loadings (5 wt.% and 20 wt.%) were characterized by TEM, TPR and XRD, and tested for toluene hydrogenation activity. The particle size and number of active sites were dependent on the support and metal loading but these factors could not be directly related to activity. The onset reduction temperature of the surface oxides did, however, relate directly to activity. As the onset reduction temperature decreased the activity increased. The ease of reducibility was affected by both metal-support interactions and the degree of Ni oxide reduction in the particles. The particle size by itself did not correlate with the reducibility of surface Ni sites and the Ni dispersion was not improved by stronger metal-support interactions.Acknowledgements
Funding from Suncor Energy Inc. for this project is gratefully acknowledged.References
- Z. R. Ismagilov, R. A. Shkrabina and N. A. Koryabkina, Catal. Today, 1999, 47, 51 CrossRef CAS.
- T.-J. Ha, H.-H. Park, E. S. Kang, S. Shin and H. H. Cho, J. Colloid Interface Sci., 2010, 345, 120 CrossRef CAS.
- C. Ruberto, PhD Thesis, Göteborg University, 2001 Search PubMed.
- Y. Yourdshahyan, C. Ruberto, M. halvarsson, L. Bengtsson, V. Langer and B. I. Lundqvist, J. Am. Ceram. Soc., 1999, 82, 1365 CAS.
- S.-D. Mo, Y.-N. Xu and W.-Y. Ching, J. Am. Ceram. Soc., 1997, 80, 1193 CAS.
- C. H. Shek, J. K. L. Lai, T. S. Gu and G. M. Lin, Nanostruct. Mater., 1997, 8, 605 CrossRef CAS.
- Y. Wang, S. Bhandari, A. Mitra, S. parkin, J. Moore and D. A. Atwood, Z. Anorg. Allg. Chem., 2005, 631, 2937 CrossRef CAS.
- C. Meephoka, C. Chaisuk, P. Samparnpiboon and P. Praserthdam, Catal. Commun., 2008, 9, 546 CrossRef CAS.
- K. V. R. Chary, P. V. R. Rao and V. V. Rao, Catal. Commun., 2008, 9, 886 CrossRef CAS.
- F.-W. Chang, M.-T. Tsay and M.-S. Kuo, Thermochim. Acta, 2002, 386, 161 CrossRef CAS.
- Q. Yu, L. Liu, L. Dong, D. Li, B. Liu, F. Gao. K. Sun, L. Dong and Y. Chen, Appl. Catal., B, 2010, 96, 350 CrossRef CAS.
- B. Adnađević and B. Janković, Phys. B, 2008, 403, 4132 CrossRef CAS.
- B. Delmon, in Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knözinger and J. Weitkamp, Wiley-VCH, New York, 1997, pp. 264–277 Search PubMed.
- G. Li, L. Hu and J. M. Hill, Appl. Catal., A, 2006, 301, 16 CrossRef CAS.
- Y.-S. Oh, H.-S. Roh, K.-W. Jun and Y.-S. Baek, Int. J. Hydrogen Energy, 2003, 28, 1387 CrossRef CAS.
- F. Li, X. Yi, J. Zheng, H. Jin and W. Fang, Catal. Commun., 2009, 11, 266 CrossRef CAS.
- J. Choi, C. B. Shin and D. J. Suh, J. Mater. Chem., 2009, 19, 7704 RSC.
- M. García-Diéguez, I. S. Pieta, M. C. Herrera, M. A. Larrubia and L. J. Alemany, Appl. Catal., A, 2010, 377, 191 CrossRef CAS.
- M. Xue, S. Hu, H. Chen, Y. Fu and J. Shen, Catal. Commun., 2011, 12, 332 CrossRef CAS.
- S. Shylesh, S. P. Mirajkar and A. P. Singh, J. Mol. Catal. A: Chem., 2005, 239, 57 CrossRef CAS.
- J. Choi, C. B. Shin and D. J. Suh, Catal. Commun., 2008, 9, 880 CrossRef CAS.
- K. Kinoshita, in Electrochemical oxygen technology, Wiley-Interscience, New York, 1992, p. 44 Search PubMed.
- G. Li, Q. Wang, D. Li, X. Lu and J. He, Mater. Chem. Phys., 2009, 114, 746 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
