Synthesis, catalysis, surface chemistry and structure of bimetallic nanocatalysts†
Franklin (Feng)
Tao
*
Department of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN, USA 46556. E-mail: ftao@nd.edu
 Franklin (Feng) Tao | Franklin (Feng) Tao joined the Department of Chemistry and Biochemistry at University of Notre Dame as a tenure-track assistant professor in 2010 after obtaining a PhD in chemistry from Princeton University followed by a postdoctoral fellowship at Lawrence Berkeley National Lab and University of California at Berkeley. He is currently leading a research group interested in catalysis, energy science, and nanoscience. His group synthesizes nanocatalysts and performs in situ and operando studies of catalytic reactions for efficient energy conversion and selective chemical transformation using in-house ambient pressure XPS and ambient pressure high temperature STM available in his group, and other in situ and operando techniques through collaborations. He has published about 80 peer-reviewed research articles in international journals and two books. He serves on editorial boards or advisory boards of several journals including Chemical Society Reviews and Catalysis Science and Technology (Royal Society of Chemistry), and Scientific Reports of Nature Publishing Group. |
Studies of bimetallic catalysts can be tracked back to the early 1960s in the Exxon Research and Engineering Company. The term “bimetallic clusters” was introduced by John H. Sinfelt in the early 1980s to refer to highly dispersed bimetallic entities present on the surface of a support such as silica or alumina.1 The main bimetallic catalysts at that time included Ni–Cu, Ru–Cu, Os–Cu, Pt–Ir, and Pt–Ru. The library of bimetallic catalysts has been significantly enriched in the past decades. Bimetallic catalysts have been one of the major categories of catalysts in heterogeneous catalysis.2 Fundamental studies of the synthesis, catalysis, surface chemistry and structure of bimetallic catalysts have been a fast-growing and exciting field of heterogeneous catalysis for energy conversion and chemical transformations.
Adding a second metal (here called the guest metal) to the first metal (called the host metal) can tune catalytic performances (activity, selectivity, durability, etc.) through modification of electronic and/or structural factors. A bimetallic catalyst is widely defined as a catalyst crystallite which consists of two metal components. The constituent metals can form an alloy, intermetallic, or nanocomposited structure. Alloy catalysts include bulk alloy, surface alloy, and near surface alloy. Nanocomposited structures include core–shell structured bimetallic nanoparticles, nanodendrites, and others.
Due to the diversity of bimetallic catalysts, tuning catalytic performance of a host metal could be performed through (a) an ensemble or geometric effect, in which the coordination of atoms of a guest metal to an atom of the host metal on the surface provides new geometries of active sites, (b) the electronic or ligand effect, wherein the addition of a guest metal alters the electron properties of the active sites of the host metal by electron transfer between guest and host metals. A concept to evaluate the influence of a guest metal on the host metal is the d-band center of the host metal.1,3 In most cases, the difference in catalytic performance between a bimetallic catalyst and a monometallic catalyst of the host metal can be rationalized through an electronic and/or geometric effect.4 Unfortunately, it is quite challenging to distinguish the two effects if both of them have a role. From the catalytic point of view, there could be another effect of a formed bimetallic catalyst which can be understood as a synergetic effect or bi-functional effect. In this case, atoms of the two metals are necessary parts of a catalytic site and thus play a unique role such as adsorption for different reactants or different intermediates.
Formation of a bimetallic catalyst exhibits the feature of continual tuning through changes in composition of the host and guest metals and flexible modification of the electronic and/or geometric structure of bimetallic catalysts through synthesis. This largely enhances the capability in tuning the catalytic performance. Thus, bimetallic catalysts have the capability of improving catalytic activity, enhancing catalytic selectivity, increasing catalytic stability, and cutting the cost of catalysts.
The spectacular advances in the synthesis of bimetallic nanocatalysts with wet chemistry in the past decade have offered numerous methods and protocols toward successful syntheses of bimetallic catalysts with well-controlled shape, size, and composition. Xia et al. (DOI: 10.1039/C2CS35173K), Tao, Zhang et al. (DOI: 10.1039/C2CS35184F), and Yang et al. (DOI: 10.1039/C2CS35189G) reviewed and summarized the main advances in the synthesis of bimetallic nanocatalysts through colloidal chemistry. Conventional methods for preparation of bimetallic catalysts are summarized by Wang, Sun et al. (DOI: 10.1039/C2CS35201J). Other syntheses through wet chemistry are discussed by Hutchings et al. (DOI: 10.1039/C2CS35296F) and Dumesic et al. (DOI: 10.1039/C2CS35188A). Chen et al. (DOI: 10.1039/C2CS35165J) described the preparation of carbide-based bimetallic model catalysts using physical vapor deposition. Goodman and Gao (DOI: 10.1039/C2CS35160A) discussed the preparation of bimetallic model catalysts in vacuum.
Bimetallic catalysts are widely used in most catalytic reactions of chemical industry and many processes in energy science and technology including oxygen reduction reaction in fuel cell and battery technology, and chemical transformation. Dumesic et al. (DOI: 10.1039/C2CS35188A) reviewed the promotion effects of bimetallic catalysts in biomass conversion; Hutchings et al. (DOI: 10.1039/C2CS35296F) reviewed the application of bimetallic catalysts to selective oxidation and other process of chemical transformation; Wang, Sun et al. (DOI: 10.1039/C2CS35201J) summarized and reviewed the production of hydrogen through different catalytic reactions on bimetallic catalysts. Catalytic activity and selectivity can be tuned through formation of bimetallic nanocatalysts. Tao, Qiao, Zong et al. (DOI: 10.1039/C2CS35182J) reviewed the enhancement of catalytic performances of reduced metal–metalloid amorphous alloys in different catalytic reactions. Tong (DOI: 10.1039/C2CS35381D) reviewed the promotion of catalytic activity in heterogeneous catalysis, particularly electrocatalysis on bimetallic catalysts.
The goal of fundamental studies of bimetallic catalysts is the understanding of catalytic mechanisms on surfaces of bimetallic catalysts at the molecular or even atomic level. Characterization of bimetallic catalysts is a critical step in understanding catalytic mechanisms and therefore designing new bimetallic catalysts. Compared to monometallic catalysts, characterization of bimetallic catalysts at nano or atomic scale is not straightforward since adding a guest metal in fact makes the characterization of bimetallic catalysts more challenging. Under the same conditions, the surface composition, oxidation state, and electronic state of a bimetallic system are typically quite different from those of a pure host metal. Electron spectroscopy,5 X-ray absorption spectroscopy,3,4 vibrational spectroscopy,6,7 electron microscopy,8 and scanning tunneling microscopy9 are powerful techniques to analyze catalyst composition, identify oxidation state and electronic state, and visualize surface structure at the atomic level. Frenkel (DOI: 10.1039/C2CS35174A) reviewed characterization of bimetallic catalysts with X-ray-based spectroscopy including XANES and EXAFS, and other inner shell X-ray spectroscopy (e.g., X-ray emission spectroscopy (XES), high energy resolution fluorescence detection (HERFD), resonant inelastic X-ray scattering (RIXS), X-ray diffraction pair distribution function (XRD/PDF), and small angle X-ray scattering (SAXS)). In addition, X-ray photoelectron spectroscopy is a powerful technique for measurement of composition, identification of valance state, and even interpretation of electronic states with great surface sensitivity.5,10,11 Yang et al. (DOI: 10.1039/C2CS35371G) reviewed the applications of advanced electron microscopy and related compositional and structural analyses to characterization of bimetallic nanocatalysts at the nanoscale and even atomic levels. For example, scanning transmission electron microscopy (STEM) is a very powerful technique to visualize elemental distribution of bimetallic nanocatalysts.
Recent advances in operando and in situ studies of catalysts have demonstrated that surface structure and chemistry of a catalyst under the reaction conditions or during catalysis could be quite different than that before or after the reaction or catalysis.10–27 These studies suggested that an in situ or operando study is necessary. As schematically showed in Fig. 1, there are four possibilities in terms of phase evolution of catalyst surfaces from the chemical state of the catalyst before reaction to that after reaction. For possibilities 3 and 4, it is necessary to track the surface chemistry and structure of the catalyst during catalysis. Unfortunately, it is challenging to predict which reactions follow possibilities 3 or 4. Thus, from the experimental point of view at least it tracks the surface chemistry and structure of a catalyst under the reaction conditions or during catalysis. As the constituent metals of a bimetallic catalyst typically have different surface energies and adsorption energies for a reactant, the information about the surface of the catalyst obtained through examination using vacuum-based electron spectroscopy or microscopy could be quite different from the real surface chemistry and structure of catalysts under reaction conditions or during catalysis. As the factors of surface free energy and adsorption energy could result in a segregation of the same metal as that resulting from surface free energies or a different metal, it can be challenging to simply extrapolate the surface chemistry of the catalyst in vacuum to that under the reaction conditions or during catalysis without in situ and operando studies. In addition, recent studies clearly suggested the pressure of the reactant could be another important term which restructures surfaces of catalysts.13,27 However, coupling the term of pressure to surface energy and adsorption energy of the constituent metals makes any straightforward prediction of surface composition and structure extremely challenging as the second metal makes the evolution of surface composition and structure much more complicated. The advance in surface analysis and in situ characterization in recent decade has made in situ or even operando characterization of surface chemistry and structure possible. Other than the techniques described by Frenkel (DOI: 10.1039/C2CS35174A), in situ electron spectroscopy, in situ electron microscopy, in situ scanning tunneling microscopy, and others have been applied to in situ or even operando tracking of surface chemistry and structure of bimetallic catalysts. Tao et al. (DOI: 10.1039/C2CS35185D) reviewed restructuring of bimetallic catalysts under reaction conditions or during catalysis.
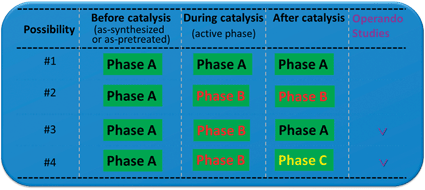 | ||
| Fig. 1 Schematic of the four different possibilities for the evolution of the catalyst surface phase from as-synthesized to that after catalysis. | ||
Bimetallic catalysts offer unique and tremendous opportunities for tuning catalytic performance and designing new catalysts for chemical transformations and energy conversion. Mechanistic understanding is typically behind the advances in synthesis and experimental evaluation of catalytic performances. A bottleneck of understanding catalytic mechanisms for bimetallic materials is a characterization of bimetallic catalysts at the atomic level under reaction conditions. However, it still remains tremendously challenging even though progress has made in the past decade. Efforts in the development of techniques and instrumentation for in situ and operando studies will be critical. In addition, development of computational methods to predict surface structure and chemistry during catalysis would certainly make screening catalysts more efficient and help understanding catalysis performed on a restructured surface. Integration of operando studies and in situ studies of surface chemistry and structure of catalysts into synthesis/preparation and measurement will greatly enhance rational design and optimization of bimetallic nanocatalysts.
Acknowledgements
FT acknowledges the efforts made by the editors, editorial board, and editorial office team of Chemical Society Reviews.References
-
J. H. Sinfelt, Bimetallic Catalysts: Discoveries, Concepts and Applications, An Exxon Monograph, 1983 Search PubMed
.
-
G. A. Somorjai and Y. Li, Introduction to surface chemistry and catalysis, Wiley, 2010 Search PubMed
.
- J. K. Norskov, T. Bligaard, J. Rossmeisl and C. H. Christensen, Nat. Chem., 2009, 1, 37–46 CrossRef CAS
.
- M. A. Newton and W. van Beek, Chem. Soc. Rev., 2010, 39, 4845–4863 RSC
.
- M. Salmeron and R. Schlogl, Surf. Sci. Rep., 2008, 63, 169–199 CrossRef CAS
.
- G. A. Somorjai, Chem. Rev., 1996, 96, 1223–1235 CrossRef CAS
.
- P. C. Stair, Curr. Opin. Solid State Mater. Sci., 2001, 5, 365–369 CrossRef CAS
.
- E. V. Orlova and H. R. Saibil, Chem. Rev., 2011, 111, 7710–7748 CrossRef CAS
.
-
R. Otero, F. Rosei and F. Besenbacher, in Annual Review of Physical Chemistry, 2006, vol. 57, pp. 497–525 Search PubMed
.
- F. Tao, M. E. Grass, Y. W. Zhang, D. R. Butcher, J. R. Renzas, Z. Liu, J. Y. Chung, B. S. Mun, M. Salmeron and G. A. Somorjai, Science, 2008, 322, 932–934 CrossRef CAS
.
- F. Tao and M. Salmeron, Science, 2011, 331, 171–174 CrossRef CAS
.
- J. Zhang, K. Sasaki, E. Sutter and R. R. Adzic, Science, 2007, 315, 220–222 CrossRef CAS
.
- F. Tao, S. Dag, L. W. Wang, Z. Liu, D. R. Butcher, H. Bluhm, M. Salmeron and G. A. Somorjai, Science, 2010, 327, 850–853 CrossRef CAS
.
- M. S. Altman, Science, 2010, 327, 789–790 CrossRef CAS
.
- H. Zheng, R. K. Smith, Y.-W. Jun, C. Kisielowski, U. Dahmen and A. P. Alivisatos, Science, 2009, 324, 1309–1312 CrossRef CAS
.
- P. L. Hansen, J. B. Wagner, S. Helveg, J. R. Rostrup-Nielsen, B. S. Clausen and H. Topsoe, Science, 2002, 295, 2053–2055 CrossRef CAS
.
- G. Ketteler, D. F. Ogletree, H. Bluhm, H. J. Liu, E. L. D. Hebenstreit and M. Salmeron, J. Am. Chem. Soc., 2005, 127, 18269–18273 CrossRef CAS
.
- E. K. Vestergaard, R. T. Vang, J. Knudsen, T. M. Pedersen, T. An, E. Laegsgaard, I. Stensgaard, B. Hammer and F. Besenbacher, Phys. Rev. Lett., 2005, 95, 126101 CrossRef
.
- E. de Smit, I. Swart, J. F. Creemer, G. H. Hoveling, M. K. Gilles, T. Tyliszczak, P. J. Kooyman, H. W. Zandbergen, C. Morin, B. M. Weckhuysen and F. M. F. de Groot, Nature, 2008, 456, U222–U239 CrossRef
.
- J. A. Rodriguez, S. Ma, P. Liu, J. Hrbek, J. Evans and M. Perez, Science, 2007, 318, 1757–1760 CrossRef CAS
.
- K. Liu, A. Wang, W. Zhang, J. Wang, Y. Huang, J. Shen and T. Zhang, J. Phys. Chem. C, 2010, 114, 8533–8541 CAS
.
- M. J. Williamson, R. M. Tromp, P. M. Vereecken, R. Hull and F. M. Ross, Nat. Mater., 2003, 2, 532–536 CrossRef CAS
.
- P. A. Crozier, R. Wang and R. Sharma, Ultramicroscopy, 2008, 108, 1432–1440 CrossRef CAS
.
- S. Helveg, C. Lopez-Cartes, J. Sehested, P. L. Hansen, B. S. Clausen, J. R. Rostrup-Nielsen, F. Abild-Pedersen and J. K. Norskov, Nature, 2004, 427, 426–429 CrossRef CAS
.
- C. W. Jones, F. Tao and M. V. Garland, ACS Catal., 2012, 2, 2444–2445 CrossRef CAS
.
- C. Wen, Y. Zhu, Y. Ye, S. Zhang, F. Cheng, Y. Liu, P. Wang and F. Tao, ACS Nano, 2012, 6, 9305–9312 CrossRef CAS
.
- H. Yoshida, Y. Kuwauchi, J. Jinschek, K. Sun, S. Tanaka, M. Kohyama, S. Shimada, M. Haruta and S. Takeda, Science, 2012, 335, 317–319 CrossRef CAS
.
Footnote |
| † Part of the bimetallic nanocatalysts themed issue. |
| This journal is © The Royal Society of Chemistry 2012 |
