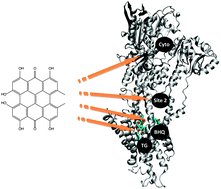
The exact cellular target for the potent anti-cancer agent hypericin has not yet been determined; this thus encourages the application of computational chemistry tools to be employed in order to provide insights that can be employed in further drug development studies. In the present study computational docking and molecular dynamics simulations are applied to investigate possible interactions between hypericin and the Ca2+ pump SERCA as proposed in the literature. Hypericin was found to bind strongly both in pockets within the transmembrane region and in the cytosolic region of the protein, although the two studied isoforms of SERCA differ slightly in their preferred binding sites. The calculated binding energies for hypericin in the four investigated sites were of the same magnitude as for thapsigargin (TG), the most potent SERCA inhibitor, or in the range between TG and di-tert-butylhydroquinone (BHQ), which is also known to possess inhibitory activity. The hydrophobic character of hypericin indicates that the molecule initially binds in the ER membrane from which it diffuses into the transmembrane region of the protein and to binding pockets therein. The transmembrane TG and BHQ binding pockets provide suitable locations for hypericin as they allow for favourable interactions with the lipid tails that surround these. High binding energies were noted for hypericin in these pockets and are expected to constitute highly possible binding sites due to their accessibility from the ER membrane. Hypericin most likely binds to both isoforms of SERCA and acts as an inhibitor or, under light irradiation, as a singlet oxygen generator that in turn degrades the protein or induces lipid peroxidation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...