Rate constants and mechanisms of intrinsically disordered proteins binding to structured targets
Abstract
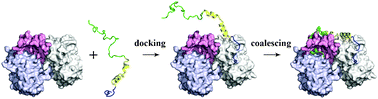
The binding of intrinsically disordered
- This article is part of the themed collection: Biophysics and biophysical chemistry in PCCP

 Please wait while we load your content...
Please wait while we load your content...