Spectral deciphering of the interaction between an intramolecular hydrogen bonded ESIPT drug, 3,5-dichlorosalicylic acid, and a model transport protein†
Abstract
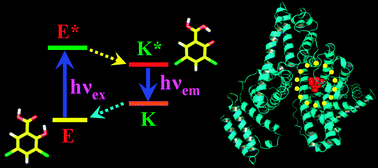
The present work demonstrates a detailed characterization of the interaction of a bio-active
- This article is part of the themed collection: Hydrogen bonding in electronically excited states

 Please wait while we load your content...
Please wait while we load your content...