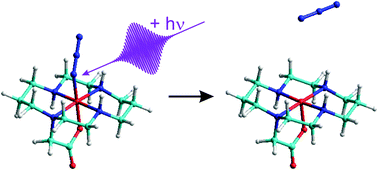
The ultrafast photo-induced primary processes of the iron-(III) azido complex, [FeIIIN3(cyclam-acetato)] PF6 (1), in acetonitrile solution at room temperature were studied using femtosecond spectroscopy with ultraviolet (UV) excitation and mid-infrared (MIR) detection. Following the absorption of a 266 nm photon, the complex undergoes an internal conversion back to the electronic doublet ground state at a time scale below 2 ps. Subsequently, the electronic ground state vibrationally cools with a characteristic time constant of 13 ps. A homolytic bond cleavage was also observed by the appearance of ground state azide radicals, which were identified by their asymmetric stretching vibration at 1659 cm−1. The azide radical recombines in a geminate fashion with the iron containing fragment within 20 ps. The cage escape leading to well separated fragments after homolytic Fe–N bond breakage was found to occur with a quantum yield of 35%. Finally, non-geminate recombination at nanosecond time scales was seen to further reduce the photolytic quantum yield to below 20% at a wavelength of 266 nm.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...