Growth of ultralong potassium titanate whiskers by the KCl flux method with metallic titanium materials†
Sayaka
Suzuki
a,
Katsuya
Teshima
*b,
Mizuho
Kiyohara
b,
Hideya
Kamikawa
c,
Kunio
Yubuta
d,
Toetsu
Shishido
d and
Shuji
Oishi
b
aDepartment of Materials Science and Engineering, Interdisciplinary Graduate School of Science and Technology, Shinshu University, 4-17-1, Wakasato, Nagano 380-8553, Japan. E-mail: 10st302g@shinshu-u.ac.jp
bDepartment of Environmental Science and Technology, Faculty of Engineering, Shinshu University, 4-17-1, Wakasato, Nagano 380-8553, Japan. E-mail: teshima@shinshu-u.ac.jp
cAqua Business Group, Business Planning Division, Yamaha Livingtec Corporation, 1370 Nishiyama-cho, Nishi-ku, Hamamatsu 432-8001, Japan. E-mail: Hideya_Kamikawa@liv.yamaha.co.jp
dInstitute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan. E-mail: yubuta@imr.tohoku.ac.jp
First published on 1st May 2012
Abstract
Well-formed, highly crystalline potassium titanate whiskers were successfully grown by the KCl flux cooling method at a holding temperature of 800 °C using metallic titanium materials. Of primary importance were the metallic Ti spheres and potassium chloride powders that were used as the starting materials for growth of the titanate whiskers. Ultralong K2Ti6O13 whiskers grew radially from the center of the Ti spheres by our flux technique. Cross-sectional SEM images demonstrate that the ultralong whiskers were grown on the TiO2 crystal layer, and the whisker spheres had a bilayer structure that was hollow in the center. When Ti powders were used as the starting material, spherical aggregation of K2Ti6O13 and K2Ti4O9 whiskers occurred. The product structures were different from those obtained from metallic Ti spheres. Based on SEM, EDS and XRD, the formation mechanism of the ultralong whiskers was also discussed. Furthermore, the photocatalytic activity of the whiskers was confirmed by trichloroethylene degradation under ultraviolet light irradiation.
1. Introduction
In various industrial fields, one-dimensional (1-D) structural materials, including tubes, fibers and rods, have been used because of their unique functional properties. Among 1-D materials, whiskers are generally defined as needle-shaped single crystals with a high aspect ratio, and it has a nearly theoretical strength due to its perfect geometry. There have been many studies of the fabrication of inorganic whiskers, such as ZnO, TiO2, WO3, TiB, BaF2 and SiC.1–8 In addition, we recently reported on the flux growth of various highly crystalline functional whiskers, such as Na2Ti6O13, Na2Ti3O7, K2Nb8O21, CaMoO4, Ca5F(PO4)3, Ca5Cl(PO4)3 and Ca5OH(PO4)3.9–15Several types of potassium titanates have been reported, including K2Ti2O5 and K2Ti4O9, which have layered structures, and K2Ti6O13 and K2Ti8O17, which have tunnel structures.16–33 With their unique crystal structures, these potassium titanates have attracted attention for use in applications such as water splitting and degradation of toxic substances.18–20 The layered potassium titanates also have the potential to be used as ion-exchangers and starting materials for fabricating nanomaterials (e.g., exfoliated nanosheets).21,22 K2Ti6O13 and K2Ti4O9 have been particularly well studied. The crystal structures of K2Ti6O13 and K2Ti4O9 are monoclinic with C2/m space group.34,35 K2Ti6O13 has lattice parameters of a = 1.5582 nm, b = 0.3820 nm, c = 0.9112 nm and β = 99.764°.34 Various techniques have been reported for the fabrication of K2Ti6O13 crystals, such as solid-state reaction using K2CO3 and TiO2 mixtures,23 hydrothermal reaction between Na2Ti3O7 and KOH,24 calcination of KF and TiO2 mixtures,25 and flux method using KCl as the flux.26–29 Among the previous reports on the growth of K2Ti6O13 crystals from the KCl flux, Wang et al. prepared K2Ti6O13 nanorings from a mixture of a K2C2O4–TiO2–KCl system in the presence of a nonionic surfactant.26 Du et al. obtained K2Ti6O13 nanobelts and annular nanostructures from mixtures of a KCl–TiO2 system and a K2CO3–TiO2–KCl system, respectively.27,28 Furthermore, Cheng et al. also used a mixed K2CO3–TiO2–KCl system to fabricate K2Ti6O13 nanoribbons.29 K2Ti4O9 has lattice parameters of a = 1.8250 nm, b = 0.3791 nm, c = 1.2010 nm and β = 106.40°.35 K2Ti4O9 crystals have been prepared by various techniques, such as solid-state reaction using K2CO3 and TiO2 mixtures,30,31 sol–gel method using KOC4H9 and Ti(OC3H7)4 mixtures,31 and flux method using K2MoO4 as the flux.32
Here, we report the fabrication of unique structures of potassium titanate whiskers by the KCl flux method. K2Ti6O13 and K2Ti4O9 whiskers were grown with metallic Ti spheres and powders as starting materials (Fig. 1). In our previous study, highly crystalline Na2Ti3O7 whiskers were successfully and directly grown on a metallic titanium mesh by the NaCl flux method.11 By applying this technique, we fabricated spherical structures consisting of potassium titanate whiskers in this study. Moreover, we demonstrated that the shape and surface morphology of the raw Ti materials affected the shapes and crystal phases of the synthesized whiskers.
 | ||
| Fig. 1 SEM images of raw titanium (a) sphere and (b) powders. | ||
2. Experimental
Potassium titanate whiskers were directly grown by cooling a KCl flux with two types of raw titanium materials: metallic Ti spheres (Fig. 1a) and Ti powders (Fig. 1b). The surface of the metallic Ti spheres was smoother than that of the Ti powders. In addition to the Ti materials, reagent-grade K2CO3 and KCl powders (Wako Pure Chemical Industries, Ltd.) were used as starting materials. In this study, the target material was set as K2Ti4O9; therefore, a mixture of K2CO3 powder (0.246 g) and Ti material (spheres or powder, 0.177 g) was used as a solute at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. KCl powder (9.469 g) was chosen as the flux, and the solute concentration was fixed at 1 mol%. The mixture was put into platinum crucibles with 30 cm3 capacity. After the lids were loosely closed, the crucibles were placed in an electric furnace. The crucibles were heated to 800 °C at a rate of 45 °C h−1 and held for 10 h. Next, they were cooled to 500 °C at a rate of 5 °C h−1 with the rate controlled by a cooling program. Finally, the crucibles were cooled to room temperature in the furnace. The crystal products were readily separated from the remaining flux in warm water. The obtained products were observed by scanning electron microscopy (SEM, JEOL, JMC-5700) and field emission scanning electron microscopy (FESEM, JEOL, JSM-7000F) at an accelerating voltage of 15 kV; the products were also examined by transmission electron microscopy (TEM, TOPCON, EM-002B) at 200 kV. The elements and compositions of the products were evaluated with energy-dispersive X-ray spectroscopy (EDS, JEOL, JSM-6710F) at an accelerating voltage of 15 kV and X-ray diffraction (XRD, RIGAKU, MiniflexII) with Cu-Kα radiation (λ = 0.154 nm).
4. KCl powder (9.469 g) was chosen as the flux, and the solute concentration was fixed at 1 mol%. The mixture was put into platinum crucibles with 30 cm3 capacity. After the lids were loosely closed, the crucibles were placed in an electric furnace. The crucibles were heated to 800 °C at a rate of 45 °C h−1 and held for 10 h. Next, they were cooled to 500 °C at a rate of 5 °C h−1 with the rate controlled by a cooling program. Finally, the crucibles were cooled to room temperature in the furnace. The crystal products were readily separated from the remaining flux in warm water. The obtained products were observed by scanning electron microscopy (SEM, JEOL, JMC-5700) and field emission scanning electron microscopy (FESEM, JEOL, JSM-7000F) at an accelerating voltage of 15 kV; the products were also examined by transmission electron microscopy (TEM, TOPCON, EM-002B) at 200 kV. The elements and compositions of the products were evaluated with energy-dispersive X-ray spectroscopy (EDS, JEOL, JSM-6710F) at an accelerating voltage of 15 kV and X-ray diffraction (XRD, RIGAKU, MiniflexII) with Cu-Kα radiation (λ = 0.154 nm).
The photocatalytic activities of whisker spheres were studied by photocatalytic degradation of trichloroethylene (C2HCl3, TCE) gas under ultraviolet (UV) irradiation. In the photocatalytic degradation of TCE, the whisker balls (0.4 g) grown from Ti spheres at 800 °C for 10 h (or grown from titanium powders at 800 °C for 10 h), and TCE gas (2 ml) were inserted into a quartz glass cell. The cell was sealed and irradiated with UV radiation (200 W, wavelength = 254–443 nm, SAN-EI Electric, UVF-204S). The photocatalytic degradation of TCE was confirmed by Fourier transform infrared spectroscopy (FT-IR, JASCO, FT/IR-6100).
3. Results and discussion
Potassium titanate whiskers were successfully grown by the KCl flux method using two types of raw Ti materials, spheres and powder, as shown in Fig. 2. Both products had a milky-white color. Fig. 2a, c and e show low-magnification, high-magnification and cross-sectional SEM images, respectively, of the whiskers grown using Ti spheres. The spherical shape of the raw Ti materials was maintained, and their surface changed from smooth to rough, as shown in Fig. 2a. The ultralong, well-developed whiskers with a high aspect ratio were grown from the surfaces of the Ti spheres (Fig. 2c). As shown in Fig. 2e, the whiskers grew radially from the center and were uniformly and densely packed. In addition, crystals with bulk-like shapes were observed under the whisker layer. Furthermore, the centers of the whisker spheres were hollow. Fig. 2b, d and f show low-magnification, high-magnification and cross-sectional SEM images, respectively, of the whiskers grown using Ti powder. As shown in Fig. 2b and d, the raw Ti powders changed into spherical shapes, and the surface was completely covered with numerous ultralong whiskers. The whiskers had a high aspect ratio and grew randomly and densely. No hollow or bulk-like crystal layer was observed (Fig. 2f), as was seen for the aggregated whiskers. From these SEM images, the whisker growth was remarkably dependent on the shape and surface morphology of raw Ti materials and differed between sphere and powder precursors.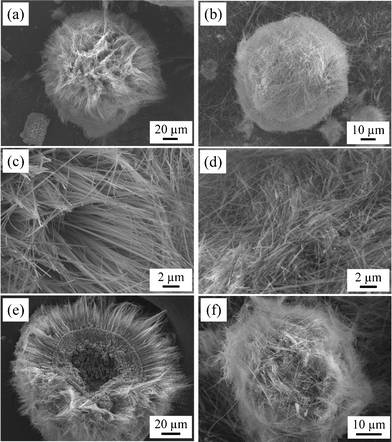 | ||
| Fig. 2 (a, b) Low-magnification, (c, d) high-magnification and (e, f) cross-sectional SEM images of potassium titanate whiskers grown from (a, c, e) Ti sphere and (b, d, f) Ti powder. | ||
Fig. 3a and b show XRD patterns for pulverized crystallites of whiskers grown using Ti spheres and powders, respectively. In both diffraction patterns, diffraction lines attributed to the raw material (Ti) and flux (KCl) were not observed. When the whiskers were grown using the Ti spheres (Fig. 3a), most of the diffraction lines were identified as K2Ti6O13 (●) (Fig. 3c).34 In addition, diffraction lines corresponding to TiO2 rutile (★) (Fig. 3d) were also observed.36 However, when the whiskers were grown using the Ti powders (Fig. 3b), the products were identified as both K2Ti6O13 (●) and K2Ti4O9 (▲) (Fig. 3e)35 while diffraction lines attributed to TiO2 rutile were not observed. For the formation of TiO2, K2Ti6O13 and K2Ti4O9 crystals from Ti, the formation processes can be described as follows:
Ti + O2 (in air) → TiO2
K2CO3 + 6TiO2 → K2Ti6O13 + CO2↑
K2CO3 + 4TiO2 → K2Ti4O9 + CO2↑
 | ||
| Fig. 3 XRD patterns for pulverized crystallites of whiskers grown from (a) Ti spheres and (b) Ti powders; (c) K2Ti6O13 (●)34 (d) TiO2 rutile (★)36 and (e) K2Ti4O9 (▲) ICDD PDF.35 | ||
Using the Ti spheres, K2Ti6O13 whiskers could be grown on the surfaces of the Ti spheres because titanium ions diffused around the Ti spheres with a uniform concentration due to their shape. Furthermore, due to their shape and surface morphology, Ti spheres were not readily dissolved in the KCl flux, unlike the Ti powders. In this study, K2CO3 powders and raw Ti spheres were mixed at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 as solutes. If Ti had completely reacted with K2CO3 (K2O), K2Ti4O9 (K
4 as solutes. If Ti had completely reacted with K2CO3 (K2O), K2Ti4O9 (K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti = 1
Ti = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) would be generated. However, a Ti-rich compound (K2Ti6O13, K
2) would be generated. However, a Ti-rich compound (K2Ti6O13, K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti = 1
Ti = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was formed, and TiO2 rutile, which formed by the oxidation of Ti and did not react with K2O, was also detected. Nevertheless, it is clear that the Ti powders were more easily dissolved in the KCl flux than the Ti spheres because K2Ti4O9 crystals grew in addition to the K2Ti6O13 crystals, and TiO2 did not remain. As clearly shown in Fig. 1, since Ti powders had rougher surface and larger specific surface area than Ti spheres, the solubility of Ti powders in KCl flux might be much larger than that of Ti spheres. For K2Ti4O9 formation, the surface of Ti powders was well dissolved in the KCl flux and well reacted with K2O due to matching molar ratio of starting materials (K
3) was formed, and TiO2 rutile, which formed by the oxidation of Ti and did not react with K2O, was also detected. Nevertheless, it is clear that the Ti powders were more easily dissolved in the KCl flux than the Ti spheres because K2Ti4O9 crystals grew in addition to the K2Ti6O13 crystals, and TiO2 did not remain. As clearly shown in Fig. 1, since Ti powders had rougher surface and larger specific surface area than Ti spheres, the solubility of Ti powders in KCl flux might be much larger than that of Ti spheres. For K2Ti4O9 formation, the surface of Ti powders was well dissolved in the KCl flux and well reacted with K2O due to matching molar ratio of starting materials (K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti = 1
Ti = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). By contrast, K2Ti6O13 (Ti-rich composition) and TiO2 (oxidized Ti powders) formations indicate that the reaction between K2O and TiO2 did not proceed fully in the KCl flux. The result of the XRD analyses confirms that the shape and surface morphology of the raw Ti materials affected not only the growth behavior of the titanate whiskers, but also the phase of the products.
2). By contrast, K2Ti6O13 (Ti-rich composition) and TiO2 (oxidized Ti powders) formations indicate that the reaction between K2O and TiO2 did not proceed fully in the KCl flux. The result of the XRD analyses confirms that the shape and surface morphology of the raw Ti materials affected not only the growth behavior of the titanate whiskers, but also the phase of the products.
Fig. 4 shows a cross-sectional FE-SEM image and the corresponding EDS mapping images of the titanate whiskers grown using the Ti spheres. A multi-layer structure consisting of the bulk crystals (inner layer) and whiskers (outer layer) was clearly observed, and the bulk crystal layer was densely covered with well-developed whiskers. EDS mapping indicated that titanium and oxygen atoms were homogeneously distributed in both crystal layers, whereas chloride atoms derived from the KCl flux were not detected. Additionally, potassium atoms were homogeneously distributed only in the whiskers. From the XRD and EDS analyses, the ultralong 1-D crystals (exterior) were identified as K2Ti6O13, and the bulk crystals (interior) were confirmed as TiO2 rutile. In the case of the aggregates fabricated using the Ti powders, it was impossible to distinguish between K2Ti6O13 and K2Ti4O9 for the synthesized whiskers, since both crystals featured the 1-D structure.
 | ||
| Fig. 4 Cross-sectional FE-SEM and EDS mapping images of potassium titanate whiskers grown from Ti sphere at 800 °C for 10 h. | ||
Next, we investigated the crystallinity of the whiskers by TEM. Fig. 5 shows a bright-field TEM image, the corresponding selected area electron diffraction (SAED) pattern, and a lattice image of K2Ti6O13 whiskers grown using the Ti spheres. The bright-field TEM image (Fig. 5a) shows that the whiskers had well-developed facets. The diffraction spots in the SAED pattern (Fig. 5b) were assigned to K2Ti6O13. Additionally, the obtained K2Ti6O13 whiskers were considered highly crystalline due to the observation of a highly ordered and sharp SAED pattern. From the lattice image (Fig. 5c), the whisker had good crystallinity because no defects were observed. TEM images of the K2Ti4O9 whiskers grown using the Ti powders are shown in electronic supplementary information (ESI 1†). The K2Ti4O9 whiskers were also confirmed to have good crystallinity.
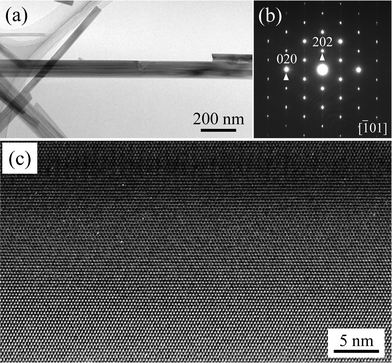 | ||
| Fig. 5 (a) Bright-field TEM image, (b) the corresponding SAED pattern and (c) the lattice image of a typical K2Ti6O13 whisker grown from Ti spheres. | ||
Fig. 6 illustrates the formation mechanism of K2Ti6O13 whisker balls grown using Ti spheres. The formation mechanism was determined based on SEM, XRD and EDS results and our previous study.11 First, the surfaces of the Ti spheres were thermally oxidized by reaction with atmospheric oxygen (Fig. 6a) and the TiO2 layer was formed on the surfaces of Ti spheres. The oxidized crystal (TiO2) layer acted as a protective layer against thermal energy, maintaining the spherical shape. Simultaneously with the formation of the TiO2 layer, the inner Ti material was dissolved by the KCl flux. TiO2 and the inner titanium ions dissolved in the solution, and the inner Ti sphere gradually became porous (Fig. 6b). The outer TiO2 layer also reacted with K2O, which was formed by the decomposition of K2CO3 (K2CO3 → K2O + CO2), and then short K2Ti6O13 whiskers with a low aspect ratio formed on the TiO2 crystal layer (Fig. 6c). Through the reaction between TiO2 (or titanium ions) and K2O, ultralong K2Ti6O13 whiskers with a high aspect ratio were grown (Fig. 6d). When the holding time was changed from 10 to 20 h, no TiO2 layer was observed, and spheres consisting of only titanate whiskers were obtained (ESI 2†), because almost all of the TiO2 layer (i.e., raw Ti material) was consumed by reacting with K2O. The basic formation mechanism of the whisker balls using the Ti powders is assumed to be the same as that using the Ti spheres; however, they had different shapes and surface morphologies, resulting in a different crystal phase and spherical shape.
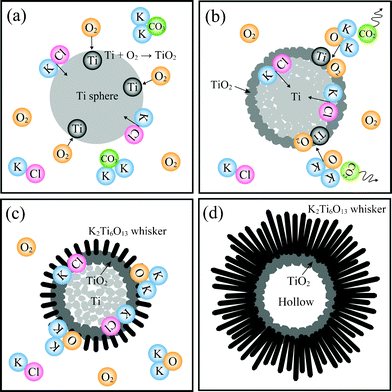 | ||
| Fig. 6 Illustrations of the formation mechanism of potassium titanate whiskers from Ti spheres. | ||
Finally, we carried out photocatalytic degradation tests for trichloroethylene (C2HCl3, TCE) using the whisker balls grown from Ti spheres (800 °C, 10 h) and Ti powders. Fig. 7 shows FT-IR spectra of the TCE gas in a packed quartz glass cell. Absorption bands due to TCE molecules were detected from 960 to 720 cm−1 in the sample that was not irradiated with UV light. The intensities of the characteristic TCE bands gradually decreased with an increase in the UV light irradiation time. Additionally, absorption bands due to CO2 (2390–2280 cm−1) and CO (2220–2060 cm−1) were observed in both samples irradiated with UV light. The generation of CO2 and CO was caused by the photocatalytic degradation of TCE.11,37 No intermediate products, such as HCl (3050–2700 cm−1), phosgene (COCl2, 1852–1768 cm−1) or dichloroacetyl chloride (DCAC, CHCl2COCl, 1768–1614 cm−1), was clearly detected in this study because the photocatalytic degradation from TCE to CO2 or CO probably occurred at a high rate. TCE was completely decomposed in 30 and 20 min by the photocatalytic effect of the titanate whisker balls grown from Ti spheres (Fig. 7a) and Ti powder (Fig. 7b), respectively. From the FT-IR spectra, a decrease in the intensity of the TCE bands and an increase in the intensity of the CO2 and CO bands were clearly observed; therefore, the titanate whiskers were confirmed to exhibit good photocatalytic activity for TCE degradation under UV light irradiation.11,37 The whisker balls containing K2Ti4O9 might also be used as cation exchangers for water purification given that K2Ti4O9 has a layered structure and ion-exchange properties.
 | ||
| Fig. 7 FT-IR spectra obtained during TCE gas photocatalytic degradation under UV light irradiation with whiskers grown from (a) Ti spheres and (b) Ti powders. | ||
4. Conclusion
Well-developed, highly crystalline and oriented K2Ti6O13 whiskers were successfully grown from titanium materials by the KCl flux cooling method at a holding temperature of 800 °C. The products grown using the Ti spheres maintained a spherical shape and had a bilayer structure consisting of an ultralong K2Ti6O13 whisker layer and a TiO2 crystal layer. In the case of the Ti powders, well-developed K2Ti6O13 and K2Ti4O9 whiskers grew randomly and densely and had no bilayer structure. Thus, it was confirmed that the shape and surface morphology of the raw Ti materials greatly affected the phase, orientation and structure of the synthesized titanate whiskers. The obtained whisker balls had diameters of several tens of micrometers, and the width of individual whiskers was several hundreds of nanometers. Therefore, the fabricated whisker balls exhibited both ease of handling and a large surface area, which are both required for applications in various fields.Acknowledgements
This research was partially supported by Industrial Technology Research Grant Program in 2009 from New Energy and Industrial Technology Development Organization (NEDO) of Japan (No. 09A18002a). A part of this work was supported by a Grant-in-Aid for JSPS Fellows (No. 23.5691) of Ministry of Education, Culture, Sports, Science and Technology, Japan.References
- J. Q. Hu, Q. Li, N. B. Wong, C. S. Lee and S. T. Lee, Chem. Mater., 2002, 14, 1216–1219 CrossRef CAS.
- B.-H. Huang, S.-Y. Chen and P. Shen, J. Phys. Chem. C, 2008, 112, 1064–1071 CAS.
- J. Ng, J. H. Pan and D. D. Sun, J. Mater. Chem., 2011, 21, 11844–11853 RSC.
- C. Klinke, J. B. Hannon, L. Gignac, K. Reuter and P. Avouris, J. Phys. Chem. B, 2005, 109, 17787–17790 CrossRef CAS.
- H. Feng, Y. Zhou, D. Jia, Q. Meng and J. Rao, Cryst. Growth Des., 2006, 6, 1626–1630 CAS.
- M. Cao, C. Hu and E. Wang, J. Am. Chem. Soc., 2003, 125, 11196–11197 CrossRef CAS.
- Z. Yang, Y. Xia and R. Mokaya, Chem. Mater., 2004, 16, 3877–3884 CrossRef CAS.
- D. C. Lim, H. S. Ahn, D. J. Choi, C. H. Wang and H. Tomokage, Surf. Coat. Technol., 2003, 168, 37–42 CrossRef CAS.
- K. Teshima, S. H. Lee, S. Murakoshi, S. Suzuki, K. Yubuta, T. Shishido, M. Endo and S. Oishi, Eur. J. Inorg. Chem., 2010, 2936–2940 CrossRef CAS.
- K. Teshima, K. Yubuta, T. Shimodaira, T. Suzuki, M. Endo, T. Shishido and S. Oishi, Cryst. Growth Des., 2008, 8, 465–469 CAS.
- K. Teshima, S. H. Lee, S. Murakoshi, S. Suzuki, M. Kiyohara, K. Yubuta, T. Shishido, M. Endo and S. Oishi, Cryst. Growth Des., 2010, 10, 2533–2540 CAS.
- K. Teshima, Y. Niina, K. Yubuta, T. Nakazawa, T. Suzuki, T. Shishido, N. Ishizawa and S. Oishi, Jpn. J. Appl. Phys., 2008, 47, 629–632 CrossRef CAS.
- K. Teshima, K. Yubuta, S. Sugiura, Y. Fujita, T. Suzuki, M. Endo, T. Shishido and S. Oishi, Cryst. Growth Des., 2006, 6, 1598–1601 CAS.
- K. Teshima, S. H. Lee, K. Yubuta, Y. Kameno, T. Suzuki, T. Shishido, M. Endo and S. Oishi, Cryst. Growth Des., 2009, 9, 3832–3834 CAS.
- K. Teshima, K. Yubuta, S. Ooi, T. Suzuki, T. Shishido and S. Oishi, Cryst. Growth Des., 2006, 6, 2538–2542 CAS.
- Q. Wang, J. S. Chung and Z. Guo, Ind. Eng. Chem. Res., 2011, 50, 8384–8388 CrossRef CAS.
- C. Liu, M. He, X. Lu, Q. Zhang and Z. Xu, Cryst. Growth Des., 2005, 5, 1399–1404 CAS.
- J. Park, J. Alloys Compd., 2010, 492, L57–L60 CrossRef CAS.
- S. Ogura, K. Sato and Y. Inoue, Phys. Chem. Chem. Phys., 2000, 2, 2449–2454 RSC.
- S. Ogura, M. Kohno, K. Sato and Y. Inoue, Phys. Chem. Chem. Phys., 1999, 1, 179–183 RSC.
- S. Tan, Y. Zhang and H. Gong, J. Water Environ. Technol., 2007, 5, 13–18 CrossRef.
- M. R. Allen, A. Thibert, E. M. Sabio, N. D. Browning, D. S. Larsen and F. E. Osterloh, Chem. Mater., 2010, 22, 1220–1228 CrossRef CAS.
- K. Shimura, H. Kawai, T. Yoshida and H. Yoshida, Chem. Commun., 2011, 47, 8958–8960 RSC.
- T. Zhang, Q. Chen and L.-M. Peng, Adv. Funct. Mater., 2008, 18, 3018–3025 CrossRef CAS.
- G. L. Li, G. H. Wang and J. M. Hong, Mater. Res. Bull., 1999, 34, 2341–2349 CrossRef CAS.
- C.-Y. Xu, Y.-Z. Liu, L. Zhen and Z. L. Wang, J. Phys. Chem. C, 2008, 112, 7547–7551 CAS.
- X. Zhang, S. Tang, L. Zhai, J. Yu, Y. Shi and Y. Du, Mater. Lett., 2009, 63, 887–889 CrossRef CAS.
- X. Zhang, S. Tang, J. Yu, L. Zhai, Y. Shi, Y. Deng and Y. Du, J. Nanosci. Nanotechnol., 2010, 10, 5111–5115 CrossRef CAS.
- L. Xu and L. Cheng, Mater. Charact., 2010, 61, 245–248 CrossRef CAS.
- H. Izawa, S. Kikkawa and M. Koizumi, J. Phys. Chem., 1982, 86, 5023–5026 CrossRef CAS.
- E. Krogh Andersen, I. G. Krogh Andersen and E. Skou, Solid State Ionics, 1988, 27, 181–187 CrossRef.
- Y. Fujiki and N. Ohta, Yogyo-Kyokai-Shi, 1980, 88, 111–116 CrossRef CAS.
- Z.-Y. Yuan, X.-B. Zhang and B.-L. Su, Appl. Phys. A: Mater. Sci. Process., 2004, 78, 1063–1066 CrossRef CAS.
- ICDD PDF 74-0275.
- ICDD PDF 32-0861.
- ICDD PDF 21-1276.
- M. D. Driessen, A. L. Goodman, T. M. Miller, G. A. Zaharias and V. H. Grassian, J. Phys. Chem. B, 1998, 102, 549–556 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ce00010e/ |
| This journal is © The Royal Society of Chemistry 2012 |
