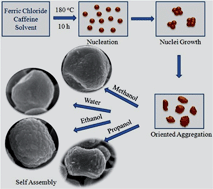
Various morphologies of hematite (α-Fe2O3) superstructures such as grape, cube, dumbbell, and microsphere shapes have been reproducibly obtained by a caffeine-assisted environmentally benign one-step hydrothermal synthesis route in the presence of different solvents. The hematite superstructures are formed by self-assembled aggregation of smaller particles of a few nanometres. It is demonstrated that systematic control over the studied experimental conditions (caffeine, solvents, concentration, reaction time, etc.) efficiently alters the structural and magnetic properties of the iron oxide nanostructures. In particular, the solvent plays a key role for overall architecture of the oxide particles under different polar conditions. Magnetic hysteresis measurements reveal that the shape of nanostructures has a remarkable effect on the magnetic properties at room temperature. Interestingly, the coercive values are much higher at room temperature than those at lower temperature for the α-Fe2O3 superstructures prepared in this work.