A dicranopteris-like Fe–Sn–Sb–P alloy as a promising anode for lithium ion batteries†
Xiao-Mei
Zheng
a,
Ling
Huang
a,
Yao
Xiao
a,
Hang
Su
a,
Gui-liang
Xu
a,
Fang
Fu
a,
Jun-Tao
Li
ab and
Shi-Gang
Sun
*a
aState Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China. E-mail: sgsun@xmu.edu.cn; Fax: +86 (0)5922180181; Tel: +86 (0)5922180181
bSchool of Energy Research, Xiamen University, Xiamen 361005, China
First published on 9th May 2012
Abstract
A novel dicranopteris-like Fe–Sn–Sb–P composite was prepared, for the first time, by electrodeposition. The quaternary Fe–Sn–Sb–P alloy of multiphase displayed an excellent cycling performance as an anode of Li ion secondary batteries.
Lithium-ion rechargeable batteries are widely used today in portable electronic devices, and have potential applications in communications, transportation and renewable-energy storage.1 In particular, to meet the need for longer-lasting electronic mobile devices and electric vehicles (EVs), intensive research has been focused on improving the electrochemical properties of anodes of Li-ion batteries in order to replace the commercial carbonaceous material which has a low theoretical energy density (372 mA h g−1).2 As a result of strong drive towards high power and energy density rechargeable batteries, there has been a growing interest in developing innovative electrode materials that can store sustainable energy with long term stability, very prolonged cycle life and meeting environmental constraints for modern electrochemistry. Metallic anode materials gained increased attention because of their high specific capacity and safety characteristics,3 such as Sn (991 mA h g−1), Sb (660 mA h g−1) etc.4 Nevertheless, these metallic anode materials exhibit drastic volume variations during the lithation–delithiation processes, resulting in cracking, crumbling and pulverization from the current collector. Consequently, their reversible capacity fades with cycling, which prohibits them from practical application. To overcome this shortcoming, the basic method is the use of multiphase composites, instead of the single phase, and mixing of active and inactive composite alloy components or of intermetallic compounds, such as amorphous tin composite oxides (ATCO),5 Sn–Fe–C,6 Sn–Sb,7 and other intermetallic alloys that show a strong structural relationship between the parent electrode and its lithiated product,8etc.
The phosphorous (group V element) has interesting crystalline structures comprising puckered layered planes that provide available paths for facile Li diffusion.9 The phosphorous has low atomic packing factor (ca. 30%), indicating large voids for the accommodation of Li ions. More importantly, it has a rich alloy phase with Li(Li3P) that provides rather a high theoretical capacity (2596 mA h g−1),10 although the electronic conductivity when it is used as an anode in Li ion batteries is poor. Fortunately, the phosphorous can form many binary and ternary alloys with other metals, which may overcome the poor electronic conductivity while maintaining the high capacity. The low intercalation potential of the phosphorous in the alloy resulting from the lower formal oxidation state of the metal, and a strong covalent character of the M–pnictogen bond lead to high-lying mixed anion–metal bands and a high degree of electron delocalization.11 Transition metal phosphides including binary compounds such as MnP4,12 FeP2,11 and few ternary compounds such as Fe–Sb–P,13 Sb–Co–P14 and Sn–Co–P,15etc. were reported recently.
In this communication, we report for the first time an innovative quaternary Fe–Sn–Sb–P composite with dicranopteris-like structure, which exhibits a good cycleability and a high energy density as an anode of lithium ion batteries. The Fe–Sn–Sb–P alloy was prepared by electrochemical deposition which is one of the most attractive methods for the synthesis of thin films with advantages of low cost and low synthesis temperature, high purity, simplicity, and environment friendliness. The electrodeposition allows also the stoichiometry, thickness, and microstructure of the deposited films to be controlled by adjusting the deposition parameters. The coarse copper foil served as a current collector. Therefore, the quaternary composite film with good conductivity and cohesion is ready to be used as an anode of lithium ion batteries and as a consequence saving the tedious process of mixing active material with carbon black and polymer binder.
The initial j–E curves of linear sweep voltammetry (LSV) recorded during Fe–P, Fe–Sb–P and Fe–Sn–Sb–P alloy electrodeposition are shown and compared in Fig. 1A. The scan rate was fixed at 5 mV s−1 and the scanning potential range was from −0.25 to −1.0 V (versus the saturated calomel electrode (SCE)). It can be seen from the black line shown in Fig. 1A that the Fe–P deposition started at −0.72 V. The current density for Fe–P deposition increases as the potential became more negative, and it rose rapidly at a potential below −0.72 V. The rapid increase in current density is attributed to electrodeposition of the Fe–P alloy together with evolution of hydrogen. With addition of Sb ions into the Fe–P plating solution, a cathodic current peak appears clearly at −0.31 V (green line), corresponding to the deposition of antimony as identified by EDX analysis shown in Fig. 1B. We could not determine any other elements except the substrate Cu and little oxygen and carbon on the surface of the deposits, which is the same as reported previously.15 The current density increases rapidly as the potential becomes more negative than −0.75 V due to co-deposition of Sb, Fe and P. With further addition of tin ions into the Fe–Sb–P plating solution, there appears another cathodic current peak at −0.58 V corresponding to the co-deposition of Sb and Sn (blue line). This co-deposition has been also identified by EDS detection shown in Fig. 1C, and the atomic ratio is Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb = 53.5
Sb = 53.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46.5. No other element was detected except the substrate Cu and little oxygen and carbon on the surface of the deposits. At potentials negative than −0.75 V, codeposition of Sb, Sn, Fe and P occurs. The comparison of 3 j–E curves in Fig. 1A illustrates that at the same potential negative than −0.7 V, the current densities for Fe–Sb–P and Fe–Sb–Sn–P deposition are larger than that for Fe–P deposition, which is ascribed to the deposition of antimony on the surface of the copper electrode. The average atomic ratio of the Fe–Sn–Sb–P composite was determined by the EDS analysis in Fig. 1D, and is Fe
46.5. No other element was detected except the substrate Cu and little oxygen and carbon on the surface of the deposits. At potentials negative than −0.75 V, codeposition of Sb, Sn, Fe and P occurs. The comparison of 3 j–E curves in Fig. 1A illustrates that at the same potential negative than −0.7 V, the current densities for Fe–Sb–P and Fe–Sb–Sn–P deposition are larger than that for Fe–P deposition, which is ascribed to the deposition of antimony on the surface of the copper electrode. The average atomic ratio of the Fe–Sn–Sb–P composite was determined by the EDS analysis in Fig. 1D, and is Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 45.13
P = 45.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.46
19.46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.83
10.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.58. A similar phenomenon that the current density of deposition of the quaternary or ternary alloy is smaller than that of the binary alloy has been also reported by A. G. Dolati et al. for electrodeposition of the Fe–Cr–Ni–Mo alloy.16 Therein, an indirect mechanism may be written as follows:17
24.58. A similar phenomenon that the current density of deposition of the quaternary or ternary alloy is smaller than that of the binary alloy has been also reported by A. G. Dolati et al. for electrodeposition of the Fe–Cr–Ni–Mo alloy.16 Therein, an indirect mechanism may be written as follows:17
| H2PO2− + 2H+ + e− → P + 2H2O | (1) |
| Sn2+ + 2e− → Sn | (2) |
| 2H+ + 2e− → H2↑ | (3) |
| Sb3+ + 3e− → Sb | (4) |
The evolution of hydrogen bubbles, an undesirable side reaction in electrodeposition, shields the reduction of metal ions on the surface sites where the hydrogen bubbles are generated, while leads the metal deposition in the vicinity of these surface sites. As a consequence, the evolution of hydrogen bubbles acts as a dynamic template,18 which results in the growth of dicranopteris-like structure.
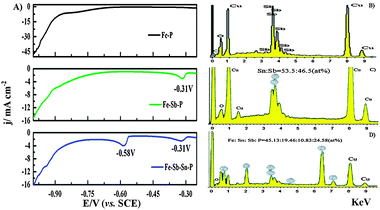 | ||
| Fig. 1 (A) The current density–cathodic potential curves of Fe–P (black line), Fe–Sb–P (green line) and Fe–Sn–Sb–P (blue line) alloy deposits by LSV. EDS spectrum of (B) Sb deposited at −0.31 V for 5 min, (C) Sn–Sb deposited at −0.58 V for 5 min and (D) deposited Fe–Sn–Sb–P alloy electrode. | ||
The SEM micrographs at different magnification (8000×, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×) of the as-deposited Fe–Sn–Sb–P alloy electrode are shown in Fig. 2A and B. A dicranopteris-like nanostructure is clearly observed. The ‘leaves’ of different lengths and widths connect along the main branch. The length of each ‘leaf’ ranges from 100 nm to 1 μm with hundreds of nanometers in width. On each leaf, there are many branches composed of many joints. Such dicranopteris-like structures as electrode material for Li ion batteries possess advantages to accommodate volume expansion of active materials during a charge–discharge cycling process. It is therefore expected that the Fe–Sn–Sb–P composite electrode will have a good endurance against the stresses and consequently a good cyclic performance. The transmission electron microscopy (TEM) image shown in Fig. 2C illustrates many branches along the main trunk. The branches range from few nanometers to hundreds of nanometers. Additionally, the selected area diffraction pattern shown in the inset to Fig. 2C indicates that the Fe–Sn–Sb–P alloy is polycrystalline. The XRD patterns of the as-deposited Fe–Sn–Sb–P alloy and amorphous Fe–P are compared in Fig. 2D. The XRD result of the as-deposited Fe–Sn–Sb–P alloy indicates clearly the presence of three different phases, i.e. the SnSb phase (2θ = 29.2°, 41.7°, 60.5° and 76.2°) (JCPDS no. 00-033-0118), the FeSb2 phase (2θ = 30.9° and 34.6°) (JCPDS no. 00-029-0129), and the Fe83P17 phase (2θ = 41.8° and 60.5°). The wide peak at around 45° can be attributed to the amorphous state of iron phosphides in the Fe–Sn–Sb–P alloy, which is the same as observed in the XRD pattern of amorphous Fe–P (indicated by red square). Besides, no other peaks are detected. Therefore, the multi-phase composite Fe–Sn–Sb–P alloy was confirmed.
000×) of the as-deposited Fe–Sn–Sb–P alloy electrode are shown in Fig. 2A and B. A dicranopteris-like nanostructure is clearly observed. The ‘leaves’ of different lengths and widths connect along the main branch. The length of each ‘leaf’ ranges from 100 nm to 1 μm with hundreds of nanometers in width. On each leaf, there are many branches composed of many joints. Such dicranopteris-like structures as electrode material for Li ion batteries possess advantages to accommodate volume expansion of active materials during a charge–discharge cycling process. It is therefore expected that the Fe–Sn–Sb–P composite electrode will have a good endurance against the stresses and consequently a good cyclic performance. The transmission electron microscopy (TEM) image shown in Fig. 2C illustrates many branches along the main trunk. The branches range from few nanometers to hundreds of nanometers. Additionally, the selected area diffraction pattern shown in the inset to Fig. 2C indicates that the Fe–Sn–Sb–P alloy is polycrystalline. The XRD patterns of the as-deposited Fe–Sn–Sb–P alloy and amorphous Fe–P are compared in Fig. 2D. The XRD result of the as-deposited Fe–Sn–Sb–P alloy indicates clearly the presence of three different phases, i.e. the SnSb phase (2θ = 29.2°, 41.7°, 60.5° and 76.2°) (JCPDS no. 00-033-0118), the FeSb2 phase (2θ = 30.9° and 34.6°) (JCPDS no. 00-029-0129), and the Fe83P17 phase (2θ = 41.8° and 60.5°). The wide peak at around 45° can be attributed to the amorphous state of iron phosphides in the Fe–Sn–Sb–P alloy, which is the same as observed in the XRD pattern of amorphous Fe–P (indicated by red square). Besides, no other peaks are detected. Therefore, the multi-phase composite Fe–Sn–Sb–P alloy was confirmed.
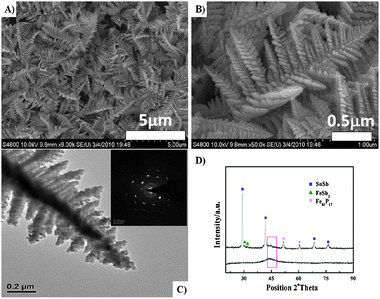 | ||
Fig. 2 SEM micrographs at different magnification of the as-deposited Fe–Sn–Sb–P alloy electrode. (A) 8000×, (B) 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× and (C) TEM of the Fe–Sn–Sb–P alloy electrode. The inset in (C) is the selected area diffraction pattern of the Fe–Sn–Sb–P alloy electrode. (D) XRD patterns of the Fe–Sn–Sb–P alloy and the amorphous Fe–P alloy. 000× and (C) TEM of the Fe–Sn–Sb–P alloy electrode. The inset in (C) is the selected area diffraction pattern of the Fe–Sn–Sb–P alloy electrode. (D) XRD patterns of the Fe–Sn–Sb–P alloy and the amorphous Fe–P alloy. | ||
Fig. 3(A) depicts discharge–charge curves of the 1st, 2nd, 3rd, 10th, 50th and 80th cycles for the dicranopteris-like Fe–Sn–Sb–P alloy electrode in a coin-type half-cell between 0.02 and 1.5 V. In the 1st cycle, the discharge (Li insertion) and charge (Li extraction) capacities of the Fe–Sn–Sb–P composite electrode attained as high as 1171 mA h g−1 and 783 mA h g−1, respectively, indicating an initial coulombic efficiency of 67%. The high charge capacity of 783 mA h g−1 is more than double that of the commercial graphite anode (372 mA h g−1). An abrupt potential drop of the first discharge observed between 1.5 and 0.80 V, which vanished in the subsequent cycles, may be caused by electrolyte decomposition to form solid electrolyte interphase (SEI) and existence of possible oxide impurities on the electrode surface. The capacity caused by this process is nonreversible. A plateau at around 0.8 V may correspond to the electrochemical formation of Li2Sb (0.82 V) and Li3Sb (0.80 V) alloys during the discharge process.19 After this plateau, the curve gradually decreases to 0.02 V, ascribing to the lithium alloying with Sn and P. There are two slopes in the charge curves. One is between 0.3 V and 0.8 V corresponding to the LixSn dealloying processes, and the other is between 0.85 and 1.1 V that is ascribed to the LixSb and LixP dealloying processes. In order to confirm these processes, the differential capacity plots (DCPs) of the first and second cycles of the Fe–Sn–Sb–P composite electrode are shown in Fig. 3B. The peaks suggest that the Fe–Sn–Sb–P composite electrode is involved in three electrochemical reactions which are in well agreement with those of the previous literature,7i.e. (1) the reaction between Sb and Li (shown by the peaks at around 0.80 V (during discharge) and 1.0 V (during charge)), (2) the reaction between Sn and Li (indicated by the peaks between 0.3 and 0.7 V (during discharge) and 0.3 and 0.8 V (during charge), marked by the dotted frames), and (3) the reaction between P and Li (confirmed by the peaks at 0.65 V and 0.02 V (discharge) and 1.07 V (charge)). Also, this is in well agreement with analysis of the charge–discharge curves.
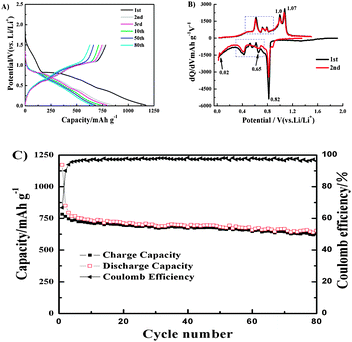 | ||
| Fig. 3 Electrochemical properties of the Fe–Sn–Sb–P alloy electrode. (A) The discharge and charge voltage profiles. (B) The differential capacity plot (DCP) of the first and second cycles and (C) Cycle behavior of the Fe–Sn–Sb–P alloy electrode at a current density of 100 mA g−1 and at a cut-off voltage of 0.02–1.5 V (vs. Li/Li+). | ||
Fig. 3(C) presents the cycle performance and coulombic efficiency of the dicranopteris-like Fe–Sn–Sb–P alloy electrode at a current of 100 mA g−1. The Fe–Sn–Sb–P electrode exhibited a stable and reversible specific capacity of approximately 627 mA h g−1, which kept 80% of the first charge capacity over 80 cycles and is superior to those of Fe–Sb–P and Sb–Co–P13,14 reported previously. In addition, a high coulombic efficiency (>95%) is achieved and kept stable starting from the 3rd cycle, signifying that the charge–discharge reactions of the Fe–Sn–Sb–P alloy electrode are reversible over cycles without any significant capacity fading. The high capacity and good cycleability can be attributed to the specific dicranopteris-like structure of the Fe–Sn–Sb–P alloy electrode which can accommodate the volume expansion generated during the cycling process. Moreover, the uniform distribution of active material (Sn, Sb, P) in the inactive matrix (Fe) (as shown in Fig. S1, ESI†) is helpful for improving the electrochemical performance.
In conclusion, a novel quaternary Fe–Sn–Sb–P composite electrode with dicranopteris-like structure was prepared for the first time. The synthesis method of electrodeposition has obvious advantages. The Fe–Sn–Sb–P composite anode demonstrated an excellent electrochemical discharge/charge behavior with a good cycleability. The large capacity and good cycleability of the composite electrode were attributed to the dicranopteris-like structure which has large space between the leaves and uniform distribution of active material (Sn, Sb, P) in the inactive matrix (Fe). These results illustrated that the multi-component of the specific dicranopteris-like structure of the Fe–Sn–Sb–P alloy electrode is a good candidate for the rechargeable Li ion batteries.
This work was financially supported by the “973” program (Grant No. 209CB220102), the “863” program (Grant No. 2011AA11A254) from the MOST, and by the NSFC (Grant No. 20773102, 2093110426, 21003102, 21021002).
Notes and references
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419–2430 CrossRef CAS.
- A. K. Shukla and T. P. Kumar, Curr. Sci., 2008, 94, 314–331 CAS.
- M. Winter, J. O. Besenhard, M. E. Spahr and P. Novak, Adv. Mater., 1998, 10, 725–763 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS.
- M. M. Thackeray, J. T. Vaughey, C. S. Johnson, A. J. Kropf, R. Benedek, L. M. L. Fransson and K. Edstrom, J. Power Sources, 2003, 113, 124–130 CrossRef CAS.
- O. Mao and J. R. Dahn, J. Electrochem. Soc., 1999, 146, 414–422 CrossRef CAS.
- C.-M. Park and K.-J. Jeon, Chem. Commun., 2011, 47, 2122–2124 RSC.
- J. O. Besenhard, J. Yang and M. Winter, J. Power Sources, 1997, 68, 87–90 CrossRef CAS.
- C. M. Park, J. H. Kim, H. Kim and H. J. Sohn, Chem. Soc. Rev., 2010, 39, 3115–3141 RSC.
- C. M. Park and H. J. Sohn, Chem. Mater., 2008, 20, 3169–3173 CrossRef CAS.
- D. C. C. Silva, O. Crosnier, G. Ouvrard, J. Greedan, A. Safa-Sefat and L. F. Nazar, Electrochem. Solid-State Lett., 2003, 6, A162–A165 CrossRef CAS.
- D. C. S. Souza, Science, 2002, 296, 2012–2015 CrossRef CAS.
- L. Huang, X. M. Zheng, Y. S. Wu, L. J. Xue, F. S. Ke, G. Z. Wei and S. G. Sun, Electrochem. Commun., 2009, 11, 585–588 CrossRef CAS.
- X. M. Zheng, Y. Xiao, L. Huang, F. S. Ke, Y. He, J. T. Li, G. Z. Wei and S. G. Sun, Electrochem. Commun., 2009, 11, 1803–1806 CrossRef CAS.
- L. Huang, Y. Yang, L. J. Xue, H. B. Wei, F. S. Ke, J. T. Li and S. G. Sun, Electrochem. Commun., 2009, 11, 6–9 CrossRef CAS.
- A. G. Dolati, M. Ghorbani and A. Afshar, Surf. Coat. Technol., 2003, 166, 105–110 CrossRef CAS.
- K. Sridharan and K. Sheppard, J. Appl. Electrochem., 1997, 27, 1198–1206 CrossRef CAS.
- S. Saadat, J. Zhu, M. M. Shahjamali, S. Maleksaeedi, Y. Y. Tay, B. Y. Tay, H. H. Hng, J. Ma and Q. Yan, Chem. Commun., 2011, 47, 9849–9851 RSC.
- C.-M. Park, S. Yoon, S.-I. Lee, J.-H. Kim, J.-H. Jung and H.-J. Sohn, J. Electrochem. Soc., 2007, 154, A917 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cc32327c |
| This journal is © The Royal Society of Chemistry 2012 |
