Chiral metal–organic frameworks with tunable open channels as single-site asymmetric cyclopropanation catalysts†‡
Abstract
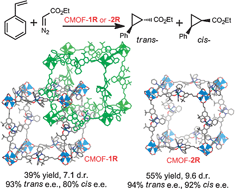
A pair of interpenetrated and non-interpenetrated chiral metal–organic frameworks with the same catalytic sites but different open channel sizes catalysed asymmetric cyclopropanation of substituted terminal
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...