Abstract
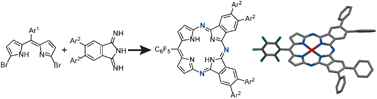
Novel triazaporphyrins were synthesized using 1,9-dibromodipyrromethene as a key starting material. These triazaporphyrins exhibit comparatively intense Soret and Q bands in the UV/vis region due to their hybrid properties between porphyrins and phthalocyanines.
- This article is part of the themed collection: Porphyrins & Phthalocyanines

 Please wait while we load your content...
Please wait while we load your content...