Regioselective zincation of indazoles using TMP2Zn and Negishi cross-coupling with aryl and heteroaryl iodides†
Andreas
Unsinn
and
Paul
Knochel
*
Ludwig Maximilians-Universität München, Department Chemie, Butenandtstraße 5-13, Haus F, 81377 München, Germany. E-mail: paul.knochel@cup.uni-muenchen.de; Fax: +49 089 2180 77680; Tel: +49 089 2180 77681
First published on 3rd February 2012
Abstract
The metalation of various SEM-protected functionalized indazoles with TMP2Zn provides 3-zincated indazoles which undergo palladium-catalyzed Negishi cross-couplings in good yields.
Indazoles are an important class of N-heterocycles which have found numerous pharmaceutical applications.1 The direct lithiation or magnesiation of indazoles at position 3 is difficult due to a facile fragmentation of these heterocycles leading to aminonitriles (Scheme 1).2
 | ||
| Scheme 1 | ||
Alternatively, 3-iodoindazoles undergo a selective I/Cu-exchange with (PhMe2CCH2)2CuLi3 leading to stable 3-cuprated indazoles which can be readily acylated.4 The lithiation,5 magnesiation,6 and zincation7 of isoindazoles (2H-indazoles) have been reported. Also the direct arylation8 of 2H-indazoles as well as the use of 3-iodoindazoles in Suzuki-9 or Stille10 cross-couplings is known.
However, the direct metalation and transition metal catalyzed arylation of 1H-indazoles has not been reported. This reaction is especially interesting due to the potential pharmaceutical activity of 3-arylated indazoles.1,11 Recently, we have described the synthesis of a kinetically highly active zinc base TMP2Zn·2MgCl2·2LiCl (1; abbreviated TMP2Zn; TMP = 2,2,6,6-tetramethylpiperidyl) which combines a high metalation activity with an excellent functional group tolerance.12,13
Herein, we wish to report that TMP2Zn (1) allows for the first time a direct metalation of a range of N-protected indazoles of type 2 under mild conditions (without concomitant ring opening) leading to bis-indazolylzincs of type 3. Their reaction with electrophiles (E) has been successfully accomplished, leading to products of type 4 (Scheme 2).
 | ||
| Scheme 2 | ||
Zinc reagents (3) react well with various electrophiles like allylic bromides and acid chlorides, but we have also found reaction conditions to perform direct arylationsvia Negishi cross-couplings14 with various aryl iodides.
Thus, preliminary experiments performed in order to find the optimal protecting group (PG) of indazole (2) showed that both a tert-butoxycarbonyl- (Boc; 2a) and a methoxymethyl protected indazole (MOM; 2b) readily react with TMP2Zn (1; THF, 25 °C, 2 h) to produce the expected bis(3-indazolyl)zinc reagents (3a–b). Copper-catalyzed trapping with various electrophiles such as ethyl 2-(bromomethyl)acrylate15 or acid chlorides provides the desired 3-functionalized indazoles (4a–c) in 72–89% yield (entries 1–3 of Table 1). A 3-arylation could be realized for the first time with the MOM-protected bis-indazolylzinc reagent (3b). Its reaction with 4-iodobenzonitrile (1.2 equiv) in the presence of 2% Pd(dba)2 (dba = dibenzylideneacetone) and 4% tfp (tfp = tri-(2-furyl)phosphine)16 at 50 °C for 8 h leads to the desired 3-arylated indazole (4d) in 76% yield. Attempts to couple bromoarenes with other catalytic systems17 were not successful. Furthermore these Negishi cross-couplings had to be performed at 50 °C. This elevated temperature proved to be a problem for the cross-coupling of further functionalized indazoles leading to partial ring opening byproducts. By switching to SEM-protected indazoles (SEM = 2-(trimethylsilyl)ethoxymethyl)18 the corresponding zinc reagents undergo Pd-catalyzed cross-couplings in high yields. Thus, the arylation of SEM-protected indazole (2c) with 4-iodobenzonitrile gives the cross-coupling product (4e) in 76% yield (entry 5). Less reactive aryl iodides, such as 4-iodoanisole (50 °C, 12 h), react now very well leading to the 3-arylated indazole (4f) in 81% yield (entry 6). A heterocyclic iodide, such as 2-iodoisoquinoline, undergoes the cross-coupling smoothly, affording the desired product (4g) in 62% yield (entry 7). This cross-coupling reaction could be extended to functionalized indazoles bearing a chlorine substituent (2d, entries 8 and 9), a bromine substituent (2e, entries 10 and 11), a methoxy group (2f, entry 12), as well as sensitive functions like a nitrile (2g, entry 13) and an ester group (2h, entry 14). The desired 3-arylated indazoles (4h–n) are produced in 45–86% yield. We verified also that these SEM-protected indazoles undergo acylation reactions. Thus, the ester substituted indazole (2h) after zincation with TMP2Zn (1) and transmetalation with CuCN·2LiCl19 reacts with benzoyl chloride leading to the 3-benzoylated indazole (4o) in 77% yield (entry 15).
| Entry | Indazole | Electrophile/conditions | Product/Yielda (%) |
|---|---|---|---|
| a Yield of isolated analytically pure product. b A transmetalation with CuCN·2LiCl (1.1 equiv) was performed. cObtained by a palladium-catalyzed cross-coupling (2% Pd(dba)2; 4% tfp; 50 °C, 6–24 h). | |||
| 1 |

|

|
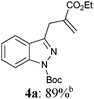
|
| 2 | PhCOCl |

|
|
| 2a | -40 to 25 °C, 2 h | ||
| 3 |

|

|

|
| 4 |

|

|
|
| 2b | |||
| 5 |

|

|

|
| 6 |
|

|
|
| 2c | |||
| 7 |

|

|
|
| 2c | |||
| 8 |

|

|

|
| 9 |

|

|
|
| 2d | |||
| 10 |

|

|

|
| 11 |

|

|
|
| 2e | |||
| 12 |

|

|

|
| 13 |

|

|

|
| 14 |

|

|

|
| 15 | PhCOCl |

|
|
| 2h | -40 to 25 °C, 2 h | ||
| 16 |

|

|

|
We have also found that the SEM protected 2H-indazole (2i) was metalated with TMP2Zn (1) under similar conditions (25 °C, 2 h) leading after copper-catalyzed acylation with thiophene-2-carbonyl chloride to the desired ketoindazole (4p) in 81% yield (entry 16).20
In summary we have reported a simple, mild and efficient method for the metalation of 1H-indazoles at position 3 with TMP2Zn (1). The resulting indazolylzincs could be arylated via Negishi cross-couplings with various aryl iodides. Applications towards the synthesis of biologically active molecules are currently being investigated in our laboratories.
We thank the Fonds der Chemischen Industrie and the European Research Council (ERC) for financial support. We also thank BASF AG (Ludwigshafen), W. C. Heraeus GmbH (Hanau) and Chemetall GmbH (Frankfurt) for the generous gift of chemicals.
Notes and references
- (a) W. Stadlbauer, Sci. Synth., 2002, 12, 227 CAS; (b) M. S. Malamas and J. Millen, J. Med. Chem., 1991, 34, 1492 CrossRef CAS; (c) S. Peruncheralathan, T. A. Khan, H. Ila and H. Junjappa, Tetrahedron, 2004, 60, 3457 CrossRef CAS; (d) C. Pabba, H.-J. Wang, S. R. Mulligan, Z.-J. Chen, T. M. Stark and B. T. Gregg, Tetrahedron Lett., 2005, 46, 7553 CrossRef CAS; (e) V. Collot, P. Dallemagne, P. R. Bovy and S. Rault, Tetrahedron, 1999, 55, 6917 CrossRef CAS; (f) J.-H. Sun, C. A. Teleha, J.-S. Yan, J. D. Rodgers and D. A. Nugiel, J. Org. Chem., 1997, 62, 5627 CrossRef CAS; (g) D. G. Batt, J. J. Petraitis, G. C. Houghton, D. P. Modi, G. A. Cain, M. H. Corjay, S. A. Mousa, P. J. Bouchard, M. S. Forsythe, P. P. Harlow, F. A. Barbera, S. M. Spitz, R. R. Wexler and P. K. Jadhav, J. Med. Chem., 2000, 43, 41 CrossRef CAS.
- (a) A. Bunnell, C. O'Yang, A. Petrica and M. J. Soth, Synth. Commun., 2006, 36, 285 CrossRef CAS; (b) J. D. Perez, G. I. Yranzo, M. A. Ferraris, J. Elguero, R. M. Claramunt and D. Sanz, Bull. Soc. Chim. Fr., 1991, 4, 592 Search PubMed; (c) W. M. Welch, C. E. Hanau and W. M. Whalen, Synthesis, 1992, 937 CrossRef CAS.
- C. Piazza and P. Knochel, Angew. Chem., 2002, 114, 3397 ( Angew. Chem., Int. Ed. , 2002 , 41 , 3263 ) CrossRef.
- X. Yang and P. Knochel, Synlett, 2004, 2303 CAS.
- (a) G. Luo, L. Chen and G. Dubowchik, J. Org. Chem., 2006, 71, 5392 CrossRef CAS; (b) A. Bunnell, C. O'Yang, A. Petrica and M. J. Soth, Synth. Commun., 2006, 36, 285 CrossRef CAS.
- C. Despotopoulou, C. Gignoux, D. McConnell and P. Knochel, Synthesis, 2009, 3661 CAS.
- B. Haag, Z. Peng and P. Knochel, Org. Lett., 2009, 11, 4270 CrossRef CAS.
- S. A. Ohnmacht, A. J. Culshaw and M. F. Greaney, Org. Lett., 2010, 12, 224 CrossRef CAS.
- A. Fraile, M. Rosario Martín, J. L. García Ruano, J. A. Díaz and E. Aranz, Tetrahedron, 2011, 67, 100 CrossRef CAS.
- F. Crestey, V. Collot, S. Stiebing and S. Rault, Synthesis, 2006, 3506 CAS.
- (a) L. A. Clutterbuck, C. Garcia Posado, C. Visintin, D. R. Riddall, B. Lancaster, P. J. Gane, J. Garthwaite and D. L. Selwood, J. Med. Chem., 2009, 52, 2694 CrossRef CAS; (b) A. Schmidt, A. Beutler and B. Snovydoch, Eur. J. Org. Chem., 2008, 4073 CrossRef CAS and references cited therein.
- (a) S. Wunderlich and P. Knochel, Angew. Chem., 2007, 119, 7829 ( Angew. Chem., Int. Ed. , 2007 , 46 , 7685 ) CrossRef; (b) Z. Dong, G. C. Clososki, S. Wunderlich, A. Unsinn, J. Li and P. Knochel, Chem.–Eur. J., 2009, 15, 457 CrossRef CAS; (c) M. Kienle, C. Dunst and P. Knochel, Org. Lett., 2009, 11, 5158 CrossRef CAS; (d) C. Dunst, M. Kienle and P. Knochel, Synthesis, 2010, 2313 CAS; (e) S. Wunderlich and P. Knochel, Org. Lett., 2008, 10, 4705 CrossRef CAS.
- For general reviews on the metallation of aromatics and heterocycles see: (a) M. C. Whisler, S. MacNeil, V. Snieckus and P. Beak, Angew. Chem., 2004, 116, 2256 ( Angew. Chem., Int. Ed. , 2004 , 43 , 2206 ) CrossRef; (b) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., 2007, 119, 3876 ( Angew. Chem., Int. Ed. , 2007 , 46 , 3802 ) CrossRef; (c) F. Chevallier and F. Mongin, Chem. Soc. Rev., 2008, 37, 595 RSC; (d) B. Haag, M. Mosrin, H. Ila, V. Malakhov and P. Knochel, Angew. Chem., 2011, 123, 9968 ( Angew. Chem., Int. Ed. , 2011 , 50 , 9794 ) CrossRef.
- (a) E. Negishi, A. O. King and N. Okukado, J. Org. Chem., 1977, 42, 1821 CrossRef CAS; (b) E. Negishi, L. F. Valente and M. Kobayashi, J. Am. Chem. Soc., 1980, 102, 3298 CrossRef CAS; (c) E. Negishi and M. Kobayashi, J. Org. Chem., 1980, 45, 5223 CrossRef; (d) E. Negishi, Acc. Chem. Res., 1982, 15, 340 CrossRef CAS; (e) E. Negishi, Angew. Chem., 2011, 123, 6870 ( Angew. Chem., Int. Ed. , 2011 , 50 , 6738 ) CrossRef.
- J. Villieras and M. Rambaud, Synthesis, 1982, 924 CrossRef CAS.
- V. Farina and B. Krishnan, J. Am. Chem. Soc., 1991, 113, 9585 CrossRef CAS.
- The following catalytic systems have been tried without success: PEPPSI-IPr; Pd(OAc)2/S-Phos; Pd(OAc)2/Ru-Phos; Pd(PPh3)4; NiCl2/dppe; Ni(acac)2/dpe.
- S. G. Pyne, M. J. Hensel and L. P. Fuchs, J. Am. Chem. Soc., 1982, 104, 5719 CrossRef CAS.
- P. Knochel, M. C. P. Yeh, S. C. Berk and J. Talbert, J. Org. Chem., 1988, 53, 2390 CrossRef CAS.
- This shows that it is in principle unnecessary to separate the isomeric 1H- and 2H-indazoles that are usually obtained as mixtures in several preparation methods.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and NMR spectra of all products. See DOI: 10.1039/c2cc17804d |
| This journal is © The Royal Society of Chemistry 2012 |
