Complex clover cross-sectioned nanotubules exist in the structure of the first uranium borate phosphate†
Shijun
Wu
ab,
Shuao
Wang
c,
Juan
Diwu
c,
Wulf
Depmeier
a,
Thomas
Malcherek
d,
Evgeny V.
Alekseev
*e and
Thomas E.
Albrecht-Schmitt
*c
aDepartment of Crystallography, University of Kiel, 24118 Kiel, Germany
bGuangzhou Institute of Geochemistry, Chinese Academy of Sciences, 510640 Guangzhou, China
cDepartment of Civil Engineering and Geological Sciences and Department of Chemistry and Biochemistry, 156 Fitzpatrick Hall, University of Notre Dame, Notre Dame, Indiana 46556, USA. E-mail: talbrec1@nd.edu
dMineralogisch-Petrographisches Institut, Universität Hamburg, 20146 Hamburg, Germany
eForschungszentrum Jülich GmbH, Institute for Energy and Climate Research (IEK-6), 52428 Jülich, Germany. E-mail: e.alekseev@fz-juelich.de
First published on 10th January 2012
Abstract
An actinide borate phosphate was prepared via a high temperature solid-state reaction. This phase exhibits unprecedented complex inorganic nanotubular fragments with an external diameter of ∼2 × 2 nm. The nanotubular aggregates are based on borate tubes where the exterior of the tubes is decorated with UO2(PO4)3 moieties to form a complex shape with a cross-section similar to the clover cross.
Nanostructured phases have been the focus of considerable interest since the discovery of carbon nanotubes.1 Numerous different topologies with nano-scale features have been found in the past two decades: nanotubes, nanorods, nanospheres, etc. Nanotubes are especially interesting owing to their unique chemical and physical properties.2 Most of these phases are not periodically ordered and are present in forms of fully or partially disordered arrays. Novel crystalline materials based on nanotubular fragments were observed recently in metal–organic hybrid materials and purely inorganic compounds.3 The family of inorganic phases based on nanotubular fragments is relatively small. It includes Na2V3O7,3d K5[(UO2)3(SeO4)5](NO3)(H2O)3.5,3e SbPS4,3f and Na2EuSiSe4.3g In addition, two organically-templated hybrid phases were reported with the following compositions: (C4H12N)14[(UO2)10(SeO4)17(H2O)]3h and (H3O)2K[(H3O)@([18]crown-6)][(UO2)3(SeO4)5](H2O)4.3i The nanotubules in the aforementioned phases possess classical tubular shapes with spherical, elliptical, or square-like cross-sections. The outer diameter of the tubes varies from 1 to 3.5 nm with internal dimensions from 0.5 to 1.5 nm. In an attempt to synthesize crystalline phases that might exist in vitrified nuclear waste,4 we synthesized a complex uranium(VI) borate phosphate, Ba5[(UO2)(PO4)3(B5O9)]·nH2O (BaBPU1).‡ This compound has an unprecedented structure with nanotubular fragments. Borophosphates form a large group of inorganic phases with borate groups (triangles or tetrahedra) that are directly linked to PO4 groups. Borate phosphates are rather rare, and contain isolated borate and phosphate groups.5 There are no known actinide borophosphates or borate phosphates.
Crystals of BaBPU1 were obtained from a high-temperature solid-state reaction, and characterized by X-ray diffraction and by several spectroscopic methods. A view of the BaBPU1 crystal structure is shown in Fig. 1a.
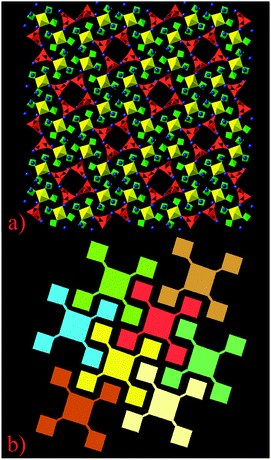 | ||
| Fig. 1 View of BaBPU1 (a) and its schematic representation (see the text for details) (b). U polyhedra are shown in yellow, phosphate tetrahedra are shown in green, borate groups in red and Ba atoms are in blue (the color scheme refers to parts (a) and (b) only). Projection on (001). | ||
The structure of BaBPU1 is based on complex nanotubular fragments with the composition of [(UO2)(PO4)3(B5O9)]1∞− linked by Ba2+ cations into a quasi 3D structure. The nanotubes have a complex shape that can be described as having a four-leafed clover cross-section. The external dimensions of the nanotubules are ∼2 × 2 nm, with an internal size approximately ∼0.6 × 0.6 nm (measured from atom centers). Two main fragments can be separated within the structure of the nanotubules in BaBPU1. The first is a borate tubular porous net (shown in red in Fig. 2). The borate net is based on the pentaborate [B5O11] anion, which can be viewed as a fundamental building block (FBB). The FBBs are linked via corner-sharing into polymeric tubules with a complex topology. The borate tubes have an external size of 1 × 1 nm. Water molecules occupy positions within the borate tube channels. The internal diameter is large enough to absorb water molecules directly from the air under standard conditions. The tubes are open from the sides and have nine-membered windows with an approximate size of 5 × 6.5 Å. The Ba2+ cations reside in the pores. A skeletal view demonstrates that the topology of the nanotubules body in BaBPU1 is similar to those found in organic and metal–organic nanotubular phases with side pores.3a,b Other inorganic nanotubes do not have pores on the sides. The borate nanotubular fragment in BaBPU1 is the only known example from borate chemistry to date.
 | ||
| Fig. 2 The structure of nanotubules in BaBPU1. The polyhedral and skeletal view of side (a and b) and top projections (c and d). Colours are the same with those in Fig. 1, water molecules are light-blue. | ||
The second fragment of this complex nanotubule is the UO2(PO4)3 cluster (UO6 shown in yellow and PO4 in green in Fig. 2). This fragment is quite well-known in uranyl phosphate chemistry and is based on almost linear uranyl groups corner-shared with PO4 tetrahedra. The uranyl groups are surrounded by only phosphate tetrahedra in all known uranyl phosphate-based materials. However, in BaBPU1, the fourth PO4 group is substituted by one BO3 triangle from the polyborate tubules.
The U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond distances in the uranyl groups are approximately 1.812(4) Å, whereas the equatorial bonds range from 2.159(44) Å to 2.322(4) Å. The U–O bond to the coordinating oxygen atom from the BO3 triangle has the shortest distance of 2.159(4) Å. The bonds in the PO4 and BO4 tetrahedra are in the ranges of 1.511(4)–1.581(4) Å and 1.414(8)–1.497(8) Å, respectively. The bonds in BO3 triangles are shorter and occur from 1.323(8)–1.395(8) Å.
O bond distances in the uranyl groups are approximately 1.812(4) Å, whereas the equatorial bonds range from 2.159(44) Å to 2.322(4) Å. The U–O bond to the coordinating oxygen atom from the BO3 triangle has the shortest distance of 2.159(4) Å. The bonds in the PO4 and BO4 tetrahedra are in the ranges of 1.511(4)–1.581(4) Å and 1.414(8)–1.497(8) Å, respectively. The bonds in BO3 triangles are shorter and occur from 1.323(8)–1.395(8) Å.
The formation of the nanotubular fragment in BaBPU1 from simple groups to a complex aggregate is schematically shown in Fig. 3. The FBBs of polyborate nanotubes are [B5O11] pentamers shown in Fig. 3a. This fragment is based on three BO3 triangles corner-linked with two BO4 tetrahedra, and can be described as 3Δ2□: 〈Δ2□〉 − 〈2Δ□〉 according to the borate classification system.6 Corner-sharing polymerization of such groups gives a hypothetical 2D sheet with the structure shown in Fig. 3b. The layer has nine-membered holes similar to those we observed in recently described actinide borates.4 The sheet topology has been known in borate chemistry for natural biringuccite and several synthetic compounds.7 The process of converting layers into tubes can be described as simple rolling up. A similar process was described for vanadate nanotubes and was proposed for several other inorganic nanotubular materials.3,8 The resulting borate nanotubules (Fig. 3d) are surrounded by UO2(PO4)3 groups via their interaction with the external BO3 triangles (Fig. 3c). Those UO2(PO4)3 clusters facing each other (the angle between them is 180°) are shifted by ½c in a relation to the neighbouring groups (90°). This shift results in a stepwise shape. The resulting nanotubes have a complex shape with a cut similar to the clover (Celtic) cross.
 | ||
| Fig. 3 A schematic representation of fragments hierarchy in BaBPU1 nanotubules. | ||
We suggest that water molecules residing inside the tubes are absorbed from air during cooling. This is based on the synthetic method we used (the temperature of the synthesis was 1000 °C). It is obvious that water is driven off at such high temperatures and the only way for it to enter the nanotubes is water absorption after or during the process of crystal cooling.9 The water molecules occupy half of their positions inside the tubes. They are strongly distorted because the internal diameter of the nanotubes is slightly larger than the size of H2O molecules. The presence of water in molecular form was confirmed by absorptions near 1600 cm−1 and 3500 cm−1 in the IR spectrum.
The packing mode of these complex nanotubes in the structure of BaBPU1 is unusual (Fig. 1a and b). Normally, nanotubular fragments are packed in crystal structures with hexagonal or tetragonal packing. Such simple packing is impossible in BaBPU1 because of the cross-like forms of the UO2(PO4)3 groups attached to the borate-based nanotubes. Generally, one can name the packing mode in BaBPU1 as a 3D puzzle assemblage. A schematic projection of nanotubes packing is shown in Fig. 1b (borate nanotubes and UO2(PO4)3 are shown in the form of large and small squares, respectively). Each UO2(PO4)3 group from a single nanotube is inserted into the free space between the UO2(PO4)3 groups of neighbouring nanotubes (for example yellow to red). The identical group from a third nanotube (cream colored) locks with the yellow UO2(PO4)3 unit by inserting into the free space of the yellow nanotube. Simultaneously, the yellow UO2(PO4)3 groups are locking the red ones by insertion into the free space of the pale greenish nanotube (Fig. 3c). All other nanotubes are locked in the same manner and form a zipper-like structure. Thus, the structure is stabilized not only by Ba2+ cations but also via coupling of the cross-shaped nanotubes. This makes the structure of BaBPU1 unique in the class of nanotubular based compounds.
This work was supported by Deutsche Forschungsgemeinschaft within the DE 412/43-1 and the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, Heavy Elements Program, U.S. Department of Energy, under Grant DE-SC0002215.
Notes and references
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- (a) R. H. Baughman, A. A. Zakhidov and W. A. De Heer, Science, 2002, 297, 787–792 CrossRef CAS; (b) P. Roy, S. Berger and P. Schmuki, Angew. Chem., Int. Ed., 2011, 50, 2904–2939 CrossRef CAS.
- (a) T.-T. Luo, H.-C. Wu, Y.-C. Jao, S.-M. Huang, T.-W. Tseng, Y.-S. Wen, G.-H. Lee, S.-M. Peng and K.-L. Lu, Angew. Chem., Int. Ed., 2009, 48, 9461–9464 CrossRef CAS; (b) K. Otsubo, Y. Wakabayashi, J. Ohara, S. Yamamoto, H. Matsuzaki, H. Okamoto, K. Nitta, T. Uruga and H. Kitagawa, Nat. Mater., 2011, 10, 291–295 CrossRef CAS; (c) P. Thanasekaran, T.-T. Luo, C.-H. Lee and K.-L. Lu, J. Mater. Chem., 2011, 21, 13140–13149 RSC; (d) P. Millet, J. Y. Henry, F. Mila and J. Galy, J. Solid State Chem., 1999, 147, 676–678 CrossRef CAS; (e) S. V. Krivovichev, V. Kahlenberg, R. Kaindl, E. Mersdorf, I. G. Tananaev and B. F. Myasoedov, Angew. Chem., Int. Ed., 2005, 44, 1134–1136 CrossRef CAS; (f) C. D. Malliakas and M. G. Kanatzidis, J. Am. Chem. Soc., 2006, 128, 6538–6539 CrossRef CAS; (g) A. Choudhury and P. K. Dorhout, J. Am. Chem. Soc., 2007, 129, 9270–9271 CrossRef CAS; (h) S. V. Krivovichev, V. Kahlenberg, I. G. Tananaev, R. Kaindl, E. Mersdorf and B. F. Myasoedov, J. Am. Chem. Soc., 2005, 127, 1072–1073 CrossRef CAS; (i) E. V. Alekseev, S. V. Krivovichev and W. Depmeier, Angew. Chem., Int. Ed., 2008, 47, 549–551 CrossRef CAS.
- (a) S. Wang, E. V. Alekseev, J. Diwu, W. H. Casey, B. L. Phillips, W. Depmeier and T. E. Albrecht-Schmitt, Angew. Chem., Int. Ed., 2010, 49, 1057–1060 CrossRef CAS; (b) P. Yu, S. Wang, E. V. Alekseev, W. Depmeier, T. E. Albrecht-Schmitt, B. Phillips and W. Casey, Angew. Chem., Int. Ed., 2010, 49, 5975–5977 CAS; (c) S. Wang, E. V. Alekseev, J. Ling, G. Liu, W. Depmeier and T. E. Albrecht-Schmitt, Chem. Mater., 2010, 22, 2155–2163 CrossRef CAS; (d) S. Wang, E. V. Alekseev, J. T. Stritzinger, W. Depmeier and T. E. Albrecht-Schmitt, Inorg. Chem., 2010, 49, 2948–2953 CrossRef CAS; (e) S. Wang, E. V. Alekseev, J. T. Stritzinger, W. Depmeier and T. E. Albrecht-Schmitt, Inorg. Chem., 2010, 49, 6690–6696 CrossRef CAS; (f) S. Wang, E. V. Alekseev, J. T. Stritzinger, G. Liu, W. Depmeier and T. E. Albrecht-Schmitt, Chem. Mater., 2010, 22, 5983–5991 CrossRef CAS; (g) S. Wang, E. V. Alekseev, J. Ling, S. Skanthakumar, L. Soderholm, W. Depmeier and T. E. Albrecht-Schmitt, Angew. Chem., Int. Ed., 2010, 49, 1263–1266 CrossRef CAS; (h) S. Wang, E. V. Alekseev, W. Depmeier and T. E. Albrecht-Schmitt, Chem. Commun., 2010, 46, 3955–3957 RSC; (i) S. Wang, E. V. Alekseev, H. M. Miller, W. Depmeier and T. E. Albrecht-Schmitt, Inorg. Chem., 2010, 49, 9755–9757 CrossRef CAS; (j) S. Wang, E. M. Villa, J. Diwu, E. V. Alekseev, W. Depmeier and T. E. Albrecht-Schmitt, Inorg. Chem., 2011, 50, 2527–2533 CrossRef CAS; (k) S. Wang, E. V. Alekseev, W. Depmeier and T. E. Albrecht-Schmitt, Inorg. Chem., 2011, 50, 2079–2081 CrossRef CAS; (l) S. Wang, E. V. Alekseev, J. Diwu, H. M. Miller, A. Oliver, G. Liu, W. Depmeier and T. E. Albrecht-Schmitt, Chem. Mater., 2011, 23, 2931–2939 CrossRef CAS; (m) M. J. Polinski, S. Wang, E. V. Alekseev, W. Depmeier and T. E. Albrecht-Schmitt, Angew. Chem., Int. Ed., 2011, 50, 8891–8894 CrossRef CAS.
- B. Ewald, Y.-X. Huang and R. Kniep, Z. Anorg. Allg. Chem., 2007, 633, 1517–1540 CrossRef CAS.
- P. C. Burns, J. D. Grice and F. C. Hawthorne, Can. Mineral., 1995, 33, 1131–1151 CAS.
- (a) E. Corazza, S. Menchetti and C. Sabelli, Am. Mineral., 1974, 59, 1005–1015 CAS; (b) P. M. Gasperin, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1987, C43, 2031–2033 CrossRef; (c) P. M. Gasperin, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1987, C43, 2264–2266 CrossRef.
- (a) K. S. Pillai, F. Krumeich, H.-J. Muhr, M. Niederberger and R. Nesper, Solid State Ionics, 2001, 141–142, 185–190 CrossRef CAS; (b) Y. D. Li, X. L. Li, R. R. He, J. Zhu and Z. X. Deng, J. Am. Chem. Soc., 2002, 124, 1411 CrossRef CAS; (c) C. Ye, G. Meng, Z. Jiang, Y. Wang, G. Wang and L. Zhang, J. Am. Chem. Soc., 2002, 124, 15180–15181 CrossRef CAS.
- E. V. Alekseev, S. V. Krivovichev and W. Depmeier, J. Solid State Chem., 2009, 182, 2074–2080 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: CIF file for Ba5[(UO2)(PO4)3(B5O9)](H2O)0.125. ICSD 423800. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2cc17517g |
‡ Synthesis: the crystals of Ba5[(UO2)(PO4)3(B5O9)](H2O)0.125 were obtained from a high-temperature solid state reaction. The following compounds were used as initial components: H3BO3 (61.38 mg); BPO4 (52.89 mg); UO2(NO3)2·6H2O (251.05 mg); Ba(CO3)2 (197.34 mg). The components were ground in an agate mortar, placed into a platinum crucible, heated to 1000 °C and then slowly (5 °C h−1) cooled to the room temperature. The resulting mixture consisted of BaBPU1 crystals (estimated yield 8–10%) and a glassy mass. Crystallographic data for Ba5[(UO2)(PO4)3(B5O9)](H2O)0.125: green needle, 0.28 × 0.09 × 0.08 mm, tetragonal, Mr = 1441.89, P42/n, Z = 8, a = b = 24.934(4) Å, c = 6.7732(6) Å, V = 4211.0(9) Å3 (T = 293(2) K), μ = 172.05 cm−1, NRef = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046/4643, Rint = 0.139, R1 = 0.0254 for Fo2 > 2σ(Fo2), wR2 = 0.0437 for all data. Infrared spectra of BaBPU1 exhibit strong absorption bands at 1320 and 1280 cm−1 which can be assigned to νas of BO4 and BO3 groups. The νs of BO4 and BO3 groups are in the range 600–810 cm−1. The vibrations of UO22+ groups are in the range from 848 to 966 cm−1. The bands of PO4 are in the range 1000–1066 cm−1. The presence of water is confirmed by the bands at 1633 and 3474 cm−1. The UV-vis-NIR and fluorescence spectra of BaBPU1 are also typical for the presence of UO22+ (see ESI†). 046/4643, Rint = 0.139, R1 = 0.0254 for Fo2 > 2σ(Fo2), wR2 = 0.0437 for all data. Infrared spectra of BaBPU1 exhibit strong absorption bands at 1320 and 1280 cm−1 which can be assigned to νas of BO4 and BO3 groups. The νs of BO4 and BO3 groups are in the range 600–810 cm−1. The vibrations of UO22+ groups are in the range from 848 to 966 cm−1. The bands of PO4 are in the range 1000–1066 cm−1. The presence of water is confirmed by the bands at 1633 and 3474 cm−1. The UV-vis-NIR and fluorescence spectra of BaBPU1 are also typical for the presence of UO22+ (see ESI†). |
| This journal is © The Royal Society of Chemistry 2012 |
