Hierarchical cobalt iron oxide nanoarrays as structured catalysts†
Jiaqiang
Sun
,
Yaping
Li
,
Xijun
Liu
,
Qiu
Yang
,
Junfeng
Liu
*,
Xiaoming
Sun
,
David G.
Evans
and
Xue
Duan
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, P.R. China. E-mail: ljf@mail.buct.edu.cn
First published on 10th January 2012
Abstract
Large-scale arrays of hierarchical cobalt iron oxide nanowires with preferentially exposed reactive crystal planes have been fabricated for use as structured catalysts, which showed high catalytic activity and excellent cycling stability.
Heterogeneous catalysts fabricated in a structured way play an important role in the so-called integrated approach to industrial catalysis and environmental protection, due to their small pressure drop, controllable mass transport, good thermal and mechanical properties, easy catalyst separation and recyclability, and other advantages which make them superior to conventional particle catalysts.1,2 A structured catalyst is most commonly made by applying a layer of a catalytically active component to the walls (or inside the walls) of an inert monolith structure. A homogeneous distribution of the active component on the monolith is often difficult to achieve, however.3 Additionally, lack of good adhesion of the active material to the monolith can be a problem because leaching occurs during catalytic reactions in solvents.4 The development of nanotechnology in recent decades has brought new opportunities in the exploration of high-performance catalysts. Nanoarrays, which have been demonstrated to be the optimized architecture for electronic/electrochemical electrodes,5,6 offer the possibility to fabricate novel structured catalysts with high reactivity and stability. Uniform structured nanoarrays with the catalytically active component directly grown on a metal substrate have a homogeneous distribution of the active material and good binding of the active material with the substrate, ideally allowing their catalytic properties to be maintained even under harsh reaction conditions (e.g., high temperature). In addition, the specific surface area of the catalysts can be increased by the design of a hierarchical structure,7–9 enhancing the accessibility of the reactant to the catalytic site and favoring molecular diffusion. Furthermore, shape and crystal plane effects of nanocrystal catalysts have attracted considerable attention for a wide variety of reactions.10–13 For instance, Co3O4 nanostructures with different dominant exposed planes show different catalytic activities for the combustion of methane and CO oxidation.11,12 Therefore, a uniform nanoarray with more reactive crystal planes exposed, directly grown on a metal substrate, should be an effective architecture for a structured catalyst.
Herein, we report the synthesis of hierarchical Co3−xFexO4 (x ≈ 1.2) nanowire arrays and their catalytic activity in the oxidation of alkenes by organic peroxides. The structure of the catalyst offers several advantages: first, the hierarchical structure of Co3−xFexO4 was grown directly on a metal (iron) substrate, which avoids the irregular distribution of the active component that tends to occur in any coating process. The direct contact of the nanoarrays with the underlying substrate also ensures that the catalyst is strongly anchored thus mitigating leaching during the catalytic reactions. Second, the open spaces associated with the hierarchical structure can facilitate the diffusion of gas and mass transport. Third, cobalt iron oxide arrays obtained by transformation of cobalt iron hydroxide carbonate precursors have predominantly exposed {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } planes, maximizing the presence of active trivalent cationic species at the surface. Finally, Fe doping enhances the catalytic efficiency of Co3O4. The hierarchical Co3−xFexO4 nanowire arrays showed high catalytic activity (92.2% conversion) and selectivity (64.6% for benzaldehyde) for styrene oxidation by tert-butyl hydroperoxide (TBHP). Notably, the array catalyst can be reused in the oxidation reactions with negligible loss of activity.
} planes, maximizing the presence of active trivalent cationic species at the surface. Finally, Fe doping enhances the catalytic efficiency of Co3O4. The hierarchical Co3−xFexO4 nanowire arrays showed high catalytic activity (92.2% conversion) and selectivity (64.6% for benzaldehyde) for styrene oxidation by tert-butyl hydroperoxide (TBHP). Notably, the array catalyst can be reused in the oxidation reactions with negligible loss of activity.
The hierarchical Co3−xFexO4 nanowire arrays on an iron substrate were achieved by a stepwise topotactic transformation process modified based on fabrication of Co3O4 nanoarrays on a glass substrate,14 as illustrated in Fig. 1a. The scanning electron microscopy (SEM) images in Fig. 1 clearly show the morphology evolution of the product. Hexagonal cobalt iron layered double hydroxide (CoFe-LDH) platelets oriented perpendicular to the surface were firstly grown on the iron substrate by hydrothermal reaction of Co2+ and F− ions and urea at 120 °C for 3 h (Fig. 1b and c). The hexagonal platelets are about 50 μm in edge length and approximately 1 μm in thickness. Continued reaction resulted in the formation of a secondary nanostructure of iron-doped orthorhombic cobalt hydroxide carbonate (CoFe-HC) nanowires which grew epitaxially out from the edges of the hexagonal CoFe-LDH platelets in a parallel fashion, with a length of about 4 μm and average width at the base of about 500 nm (Fig. 1d and e). These hierarchical structures exhibit a six-fold symmetry, i.e., the nanowire branches grow along six directions on the hexagonal platelets with an angle of 60° between adjacent branches. With increasing reaction time, the nanowires gradually grew longer, and finer nanowires with an average width and length of approximately 200 nm and 10 μm were obtained after 12 h, as displayed in Fig. 1f and g. The nanowires are interconnected with each other at an angle of 60°, forming knitted platelet morphology. Schematic illustrations of the crystallites are shown as insets in Fig. 1b, d and f. Along with SEM observations, X-ray diffraction (XRD) patterns recorded at different growth stages clearly reveal the evolution of composition from CoFe-LDH to CoFe-HC (Fig. S1, ESI†). All the peaks of the precursor obtained after 3 h can be indexed to the rhombohedral CoFe-LDH phase (JCPDS card, no. 50-0235). After longer reaction times, additional peaks characteristic of orthorhombic CoFe-HC appeared. The CoFe-HC phase was obtained after reaction for 12 h as illustrated in Fig. S1c (ESI†). All the peaks in the diffraction pattern have slightly larger 2θ values compared with the standard XRD pattern of cobalt carbonate hydroxide (JCPDS card, no. 48-0083), which can be attributed to the substitution by iron of some of the cobalt ions in cobalt hydroxide carbonate. High-resolution TEM (HRTEM) confirms the single-crystalline CoFe-HC nanowires grown along the [300] direction (Fig. 1h). The Fe-doping level was measured by energy dispersive spectroscopy (EDS) analysis (Fig. 1i) and X-ray photoelectron spectroscopy (XPS) (Fig. S2, ESI†), which show the atomic ratio of Co/Fe to be around 1.5/1.
 | ||
| Fig. 1 (a) Schematic mechanism of the stepwise growth process of hierarchical Co3−xFexO4 arrays on the iron substrate; SEM images of the products formed at: (b, c) 3 h; (d, e) 4 h; (f, g) 12 h; the insets of b, d and f show schematic illustrations of as-prepared crystallites; (h,i) HRTEM and EDS patterns of the as-made CoFe-HC nanowire. | ||
Cobalt iron oxide arrays were obtained by calcination of CoFe-HC in air. All the peaks in the XRD pattern shown in Fig. 2a can be indexed to the face-centered cubic (fcc) phase (JCPDS card, no. 74-3417) of a spinel Co3−xFexO4. As shown in Fig. 2b, the hierarchical structures with multiple rows of secondary nanowires grown on the major hexagonal platelets were mostly preserved after the calcination treatment. In the enlarged images, shown in Fig. 2c and d, it can be clearly seen that the nanowires have a regular rectangular cross-section with a thickness of 80 nm and width of 200 nm. From HRTEM analysis of Co3−xFexO4 nanowires (Fig. 2e and f), the morphology of the nanowire can be approximately derived, as shown in Fig. 2g. The Co3−xFexO4 nanowire grows along the [110] direction and preferentially exposes the {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } planes, the surface area of which is estimated to contribute 71% of the total surface area. EDS and XPS analyses show that the atomic ratio of Co/Fe is consistent with that in the pre-calcined precursor (Fig. S2 and S3, ESI†). Furthermore, the surface areas of the materials were measured by an electrochemical method (see ESI†), which indicates considerable increase of surface area of the material in the stepwise growth process (Table S1, ESI†).
} planes, the surface area of which is estimated to contribute 71% of the total surface area. EDS and XPS analyses show that the atomic ratio of Co/Fe is consistent with that in the pre-calcined precursor (Fig. S2 and S3, ESI†). Furthermore, the surface areas of the materials were measured by an electrochemical method (see ESI†), which indicates considerable increase of surface area of the material in the stepwise growth process (Table S1, ESI†).
 | ||
| Fig. 2 XRD (a), SEM (b, c), HRTEM (d, e, f) and FFT patterns of Co3−xFexO4; (g) schematic illustration of the Co3−xFexO4 nanowire. | ||
The oxidation of styrene to benzaldehyde was chosen as a probe reaction to study the catalytic properties of the materials. Benzaldehyde is a very important fine chemical product, which is widely used in many fields, such as medicine, dyes, flavors, and resin additives. The selective oxidation of styrene with tert-butyl hydroperoxide (TBHP) as an oxidant to produce benzaldehyde is of great interest because it is a green process.15 Spinel-type catalysts have been found to show high selectivity for styrene oxidation to give benzaldehyde.16,17 However, although they are stable and cheap, spinel-based catalysts reported in the literature are not efficient.17 Moreover, previously reported powdered spinel catalysts are not easy to separate and recycle. Fig. 3a shows the catalytic properties of the hierarchical Co3−xFexO4 nanoarrays. The conversion of styrene reached 77% after 3 h, and increased to 92.2% after 12 h. Compared with the Co3−xFexO4 nanoarray, Co3O4 nanorod arrays were also prepared by replacing the iron substrate with a glass slide and characterized by XRD, SEM and HRTEM analyses (Fig. S4, ESI†).14 Each nanorod is around 100 nm in diameter and about 500 nm in length with a sharp tip. The rod-like morphology means that the Co3O4 nanorods expose many crystal planes on their side face, which are very different from the hierarchical Co3−xFexO4 nanoarrays. Co3O4 nanorod arrays showed lower activity (27.8% styrene conversion after 3 h, and 75.1% styrene conversion after 12 h) than hierarchical Co3−xFexO4 nanoarrays. The specific rate of conversion over Co3−xFexO4 was 2.8 times higher than that over Co3O4 at 3 h, indicating that the hierarchical Co3−xFexO4 nanoarrays are significantly more active than Co3O4. Our hierarchical Co3−xFexO4 nanoarrays also show a higher catalytic conversion in the oxidation of styrene than previously reported spinel catalysts, as listed in Table S2 (ESI†). One of the advantages of the iron substrate for the Co3−xFexO4 nanoarray is that it can be molded into the desired shape for a particular catalytic reactor, such as rolling into a cylindrical form. The optical images shown in the inset of Fig. 3a illustrate that the Co3−xFexO4 array film of about 8 cm2 can be severely rolled without visible signs of degradation, revealing the good flexibility and great mechanical robustness of the Co3−xFexO4 array. It can be therefore be directly used in the catalytic reaction in the form of a structured catalyst. In order to check the recyclability of the Co3−xFexO4 nanoarray catalyst, the catalyst was repeatedly used for the oxidation of styrene. After each reaction the catalyst was directly reused under the same reaction conditions without any treatment. The conversion of styrene and the selectivity to benzaldehyde of the recycled catalyst were almost equal to the corresponding values for the fresh catalyst (Fig. 3b). SEM images of the Co3−xFexO4 nanoarray catalyst after being used nine times also confirmed that the catalytic reaction does not induce any significant structural or micro-structural changes in the Co3−xFexO4 nanoarray catalyst (Fig. S5, ESI†), indicating that it can meet the requirements of long cycle lifetime.
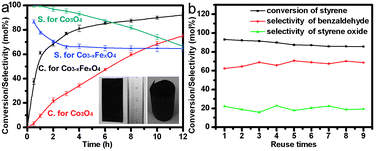 | ||
| Fig. 3 (a) Effect of varying the reaction time on styrene conversion and benzaldehyde selectivity for Co3−xFexO4 and Co3O4 catalysts, inset: optical images of film of Co3−xFexO4 nanoarrays (left) and in the form of structured catalyst (right); (b) the recyclability of the Co3−xFexO4 nanoarray structured catalyst. | ||
The hierarchical design of the Co3−xFexO4 nanoarray with more reactive crystal planes being exposed is the origin of the high catalytic activity of the material. (I) The introduction of a large number of active Co3+ sites by exposing the more active crystal planes {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } of Co3O4. From analysis of the surface atomic configurations of most common exposed planes of Co3O4 (Fig. S6, ESI†), it is known that the active Co3+ sites are located on the surface layer of the {1
} of Co3O4. From analysis of the surface atomic configurations of most common exposed planes of Co3O4 (Fig. S6, ESI†), it is known that the active Co3+ sites are located on the surface layer of the {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } and {110} planes, while the {1
} and {110} planes, while the {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1} plane does not contain octahedrally coordinated Co3+, but its sub-layers do. Furthermore, the area covered by the adjacent four oxygen atoms in the {1
1} plane does not contain octahedrally coordinated Co3+, but its sub-layers do. Furthermore, the area covered by the adjacent four oxygen atoms in the {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } planes is much larger, suggesting that the {1
} planes is much larger, suggesting that the {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } planes are more open, and the {1
} planes are more open, and the {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } planes are more reactive surfaces.11 Theoretical calculations using the density functional theory (DFT) method (see ESI†) for different surfaces also confirm that styrene and TBHP molecules interact preferably with the {1
} planes are more reactive surfaces.11 Theoretical calculations using the density functional theory (DFT) method (see ESI†) for different surfaces also confirm that styrene and TBHP molecules interact preferably with the {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) } planes, since their adsorption energies are more favorable than on other crystal planes. (II) The influence of Fe as a modifying additive. The structure of Co3−xFexO4 transforms from normal spinel into inverse spinel as x increases,18 which leads to the appearance of new active sites in the samples—Fe3+ ions in octahedral coordination increase the mobility of the reactive oxygen.19 (III) The hierarchical porous architecture of the material also contributes to the high activity and especially the good cycling stability. The micron-scale LDH nanosheets, directly grown out from the iron substrate, act as a connecting buffer between the macroscale substrate and the nanoscale nanowires, and thus can ensure efficient anchoring of the nanowires and prevent leaching during catalytic reactions. The nanosheet also provides a 3D scaffold to support the growing Co3−xFexO4 nanowires, giving a rough surface with a large surface area and preventing aggregation during the growth process and catalytic reaction. The nanosheets also afford an efficient template for the hierarchical hybrid array growth. Furthermore, compared with conventional nanowire arrays, the spaces between neighboring nanowires on the nanosheets are much larger which allows for easy gas diffusion and mass transport, resulting in a high utilization of materials.
} planes, since their adsorption energies are more favorable than on other crystal planes. (II) The influence of Fe as a modifying additive. The structure of Co3−xFexO4 transforms from normal spinel into inverse spinel as x increases,18 which leads to the appearance of new active sites in the samples—Fe3+ ions in octahedral coordination increase the mobility of the reactive oxygen.19 (III) The hierarchical porous architecture of the material also contributes to the high activity and especially the good cycling stability. The micron-scale LDH nanosheets, directly grown out from the iron substrate, act as a connecting buffer between the macroscale substrate and the nanoscale nanowires, and thus can ensure efficient anchoring of the nanowires and prevent leaching during catalytic reactions. The nanosheet also provides a 3D scaffold to support the growing Co3−xFexO4 nanowires, giving a rough surface with a large surface area and preventing aggregation during the growth process and catalytic reaction. The nanosheets also afford an efficient template for the hierarchical hybrid array growth. Furthermore, compared with conventional nanowire arrays, the spaces between neighboring nanowires on the nanosheets are much larger which allows for easy gas diffusion and mass transport, resulting in a high utilization of materials.
In summary, hierarchical Co3−xFexO4 nanoarrays have been directly grown on an iron substrate by a controllable hydrothermal process involving the epitaxial growth of cobalt iron hydroxide carbonate nanowires on an initially formed coating of CoFe-LDH nanosheets. Such architectures showed excellent catalytic activity and stability for styrene oxidation, which can be related to the preferential exposure of crystal planes with a relatively high density of active species (i.e. {1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) }), the doping of Fe in situ, and the hierarchical design of the nanoarray. This strategy can be extended to synthesize other hierarchical metal oxide arrays on metallic substrates and offers new opportunities for the design of new types of highly efficient structured catalysts.
}), the doping of Fe in situ, and the hierarchical design of the nanoarray. This strategy can be extended to synthesize other hierarchical metal oxide arrays on metallic substrates and offers new opportunities for the design of new types of highly efficient structured catalysts.
This work was financially supported by the NSFC, the Beijing Natural Science Foundation, the Program for New Century Excellent Talents in Universities, and the 973 Program (No. 2011CBA00503, 2011CB932403).
Notes and references
- V. Tomasic and F. Jovic, Appl. Catal., A, 2006, 311, 112 CrossRef CAS.
- G. Centi and S. Perathoner, Catal. Today, 2003, 79, 3 CrossRef.
- P. Sonström, M. Adam, X. Wang, M. Wilhelm, G. Grathwohl and M. Bäumer, J. Phys. Chem. C, 2010, 114, 14224 Search PubMed.
- C. M. Crudden, M. Sateesh and R. Lewis, J. Am. Chem. Soc., 2005, 127, 10045 CrossRef CAS.
- Y. Li, P. Hasin and Y. Wu, Adv. Mater., 2010, 22, 1926 CAS.
- Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265 CrossRef CAS.
- X. Huang, S. Tang, X. Mu, Y. Dai, G. Chen, Z. Zhou, F. Ruan, Z. Yang and N. Zheng, Nat. Nanotechnol., 2011, 6, 28 CrossRef CAS.
- A. T. Bell, Science, 2003, 299, 1688 CrossRef CAS.
- X. Huang, S. Tang, J. Yang, Y. Tan and N. Zheng, J. Am. Chem. Soc., 2011, 133, 15946 CrossRef CAS.
- K. Zhou, X. Wang, X. Sun, Q. Peng and Y. Li, J. Catal., 2005, 229, 206 CrossRef CAS.
- L. Hu, Q. Peng and Y. Li, J. Am. Chem. Soc., 2008, 130, 16136 CrossRef CAS.
- X. Xie, Y. Li, Z.-Q. Liu, M. Haruta and W. Shen, Nature, 2009, 458, 746 CrossRef CAS.
- X. Liu, J. Liu, Z. Chang, X. Sun and Y. Li, Catal. Commun., 2011, 12, 530 CrossRef CAS.
- J. Jiang, J. P. Liu, X. T. Huang, Y. Y. Li, R. M. Ding, X. X. Ji, Y. Y. Hu, Q. B. Chi and Z. H. Zhu, Cryst. Growth Des., 2010, 10, 70 CAS.
- S. Bose, A. Pariyar, A. N. Biswas, P. Das and P. Bandyopadhyay, J. Mol. Catal. A: Chem., 2010, 332, 1 CrossRef CAS.
- D. Guin, B. Baruwati and S. V. Manorama, J. Mol. Catal. A: Chem., 2005, 242, 26 CrossRef CAS.
- S. K. Pardeshi and R. Y. Pawar, J. Mol. Catal. A: Chem., 2011, 334, 35 CrossRef CAS.
- K. J. Kim, H. K. Kim, Y. R. Park, G. Y. Ahn, C. S. Kim and J. Y. Park, Hyperfine Interact., 2006, 169, 1363 CrossRef CAS.
- S. G. Christoskova, M. Stoyanova and M. Georgieva, Appl. Catal., A, 2001, 208, 243 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figures, characterization, catalytic reaction and theoretical calculations. See DOI: 10.1039/c2cc17368a |
| This journal is © The Royal Society of Chemistry 2012 |
