Ligand denticity controls enantiomeric preference in DNA-based asymmetric catalysis†
Abstract
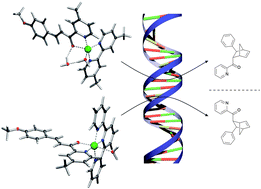
DNA-based catalysis can be used to control the enantioselectivity of copper-catalysed Diels–Alder and Friedel–Crafts reactions to produce either enantiomer of the product by changing the denticity of the
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...