Selenacalix[3]triazines: synthesis and host–guest chemistry†
Joice
Thomas
a,
Wim Van
Rossom
a,
Kristof Van
Hecke
b,
Luc Van
Meervelt
b,
Mario
Smet
a,
Wouter
Maes
ac and
Wim
Dehaen
*a
aMolecular Design and Synthesis, Department of Chemistry, Katholieke Universiteit Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: wim.dehaen@chem.kuleuven.be; Tel: +32 16327439
bBiomolecular Architecture, Department of Chemistry, Katholieke Universiteit Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium
cDesign & Synthesis of Organic Semiconductors (DSOS), Institute for Materials Research (IMO), Hasselt University, Universitaire Campus, Agoralaan – Building D, 3590 Diepenbeek, Belgium
First published on 17th October 2011
Abstract
The heteracalixarene series (N/O/S) has been expanded with Se-bridged cyclotrimeric macrocycles. Selenacalix[3]triazines were synthesized by convenient one-pot nucleophilic aromatic substitution reactions and they showed peculiar supramolecular features. The N tridentate binding pocket was capable of coordinating both copper ions and anions.
The introduction of heteroatoms replacing the methylene linkages of traditional calixarenes is an efficient approach to modulate the macrocycle's properties. The resulting heteracalixarenes are particularly attractive for applications in supramolecular chemistry since the bridging heteroatoms enable us to tune the ring size and host conformation, and provide additional binding sites towards a perfect (induced) fit of a desirable guest.1–3 Among the heteracalixarenes (mostly S, N and O, but also Si, Ge, Sn and P) the thia analogues have been studied most and they are widely recognized as receptors for small organic compounds and heavy/transition metals.1b,c On the other hand, reports on the synthesis and applications of aza- and oxacalixarenes have steadily increased during the last decade.1d–fAzacalixarene cyclooligomers have been explored as powerful hosts towards fullerene binding and for selective adsorption of CO2 in the solid state.2 Modern oxacalixarene research has mainly been focused on the development of novel synthetic procedures and postmacrocyclization modifications of the calixarenoid framework, although the enlarged scope of O-bridged calix(hetero)aromatics has triggered the first initiative towards supramolecular applications.3 Up to now, there has been no information on the expansion of heteracalixarene chemistry from the smaller groupvia elements (O/S) to the subsequent third or fourth-row “isologues” of the chalcogen series (Se/Te).
Organoselenium chemistry is intensively studied because of its potential impact on cancer prevention, inflammation protection, immune response and photodynamic therapy.4 Encouraged by the importance of natural selenoproteins, many organo-Se compounds have been designed to mimic the functions demonstrated by natural enzymes such as glutathione peroxidase and towards antithyroid drugs.5Macrocycles containing Se are attractive targets for several reasons.6 The larger atomic radius of Se leads to an increased cavity size and it may impose electronic and structural characteristics that are quite different from those of O-, N- or S-containing macrocycles. The enhanced σ-donating ability of Se can strengthen its complexation ability towards transition metals and electron acceptors. The presence of Se atoms also imposes a novel derivatization pathway by reversible oxidation to selenoxides or selenones, which enables these molecules to bind different guest species in their different oxidation states.
The peculiar features of Se considered together with the significant difference in physical properties of the heteracalixarene series prompted us to design and constructcalixarenoid macrocycles containing bridging Se atoms.6e The general synthetic strategy is based on the nucleophilic aromatic substitution (SNAr) reaction of in situ generated Se bisnucleophiles with electrophilic halogenated heterocycles. As their highly electron deficient nature ensures excellent reactivity in SNAr reactions, 2,4-dichloro-1,3,5-triazine building blocks were applied (Scheme 1).1f,g
![Synthetic pathway towards selenacalix[3]triazines 2a–c.](/image/article/2012/CC/c1cc15473g/c1cc15473g-s1.gif) | ||
| Scheme 1 Synthetic pathway towards selenacalix[3]triazines 2a–c. | ||
The first synthetic trial involved the addition of NaSeH (freshly prepared by reaction of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of Se powder and NaBH4 suspended in EtOH) to a solution of 2-butyl-4,6-dichloro-1,3,5-triazine (1a) in THF, yielding selenacalix[3]-triazine 2a in a very poor yield (4%). By optimizing the SNAr conditions the yield was increased spectacularly to 75%.7 Another profound advantage of the synthetic protocol is the easiness of purification of the cyclotrimer simply by precipitation directly from the reaction mixture in water. Curiously, only the selenacalix[3]arene macrocycle was formed under all conditions.7 To vary the substitution pattern, functional moieties were introduced prior to macrocyclization. The corresponding trimeric cyclooligomer 2b (R = OPh) was prepared in a similarly high yield (70%; Scheme 1). Under the same conditions the reaction of triazine precursor 1c (R = NEt2) and NaSeH resulted in an unsatisfactory yield of selenacalix[3]arene 2c (4%). An appreciable amount of unreacted starting material remained in the reaction mixture, which can be attributed to the (further) increase in electron density. A substantially improved conversion (55% 2c) was achieved by optimizing the concentration, reaction time and temperature.7 Calix[3]triazines 2a–c showed good solubility in a variety of organic solvents and were adequately characterized by NMR spectroscopy (1H, 13C and 77Se) and (high resolution) mass spectrometry.
2 molar ratio of Se powder and NaBH4 suspended in EtOH) to a solution of 2-butyl-4,6-dichloro-1,3,5-triazine (1a) in THF, yielding selenacalix[3]-triazine 2a in a very poor yield (4%). By optimizing the SNAr conditions the yield was increased spectacularly to 75%.7 Another profound advantage of the synthetic protocol is the easiness of purification of the cyclotrimer simply by precipitation directly from the reaction mixture in water. Curiously, only the selenacalix[3]arene macrocycle was formed under all conditions.7 To vary the substitution pattern, functional moieties were introduced prior to macrocyclization. The corresponding trimeric cyclooligomer 2b (R = OPh) was prepared in a similarly high yield (70%; Scheme 1). Under the same conditions the reaction of triazine precursor 1c (R = NEt2) and NaSeH resulted in an unsatisfactory yield of selenacalix[3]arene 2c (4%). An appreciable amount of unreacted starting material remained in the reaction mixture, which can be attributed to the (further) increase in electron density. A substantially improved conversion (55% 2c) was achieved by optimizing the concentration, reaction time and temperature.7 Calix[3]triazines 2a–c showed good solubility in a variety of organic solvents and were adequately characterized by NMR spectroscopy (1H, 13C and 77Se) and (high resolution) mass spectrometry.
The successful formation of selenacalix[3]triazines with high yield and selectivity rather than linear oligomeric or polymeric materials was achieved by carefully optimizing the appropriate SNAr reaction parameters.7 The absence of evidence for the formation of larger cyclic oligomers suggests that the trimeric macrocycles may be the thermodynamically favoured products from a reversible dynamic process of ring opening and cyclization. This contrasts the trend in oxacalixarene chemistry in which oxacalix[3]arenes have never been observed so far and the oxacalix[4]arene represents the thermodynamic sink.1e,f,3 Heteracalix[3]arenes are known in the thia- and azacalixarene field, in which they are usually obtained in small amounts from one pot reactions affording a mixture of calixarenes of different ring sizes, and they have shown peculiar supramolecular features.8
Single crystals of selenacalix[3]triazine 2c were grown to elucidate its solid-state structure. A distorted coplanar conformation was observed, i.e. the three triazine units are tilted with respect to the plane formed by the bridging Se atoms, with two of the rings inclined below the plane (12.3° and 20.5°) and one above the plane (9.3°) (Fig. 1). The macrocycles are stacked on top of each other, forming columns along the [010] direction (see ESI†).
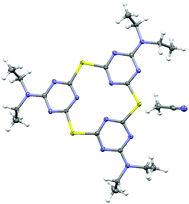 | ||
| Fig. 1 ORTEP representation of 2c, as determined by X-ray crystallography. Thermal displacement ellipsoids are shown at the 50% probability level. | ||
The most appealing property of calixarenes is their tendency to form host–guest complexes with a variety of organic and inorganic species. A limited number of (mostly azine-based) heteracalix(het)arene macrocycles have previously been shown to form complexes with metal ions, both in solution and in the solid state.2,3,8d Thiacalix[3]pyridines have been shown to bind Cu and Rhvia their pyridine lone pairs.8c,e The binding potential of the selenacalix[3]triazines has initially been evaluated using macrocycle 2c. Reaction of 2c with one equivalent of CuBr2 in THF at RT resulted in precipitation of the monomeric complex [2c–CuBr2] (3c) as a green solid in high yield (94%). The molecular structure of the complex was unambiguously determined by X-ray crystallography (Fig. 2). In comparison with the structure of the free ligand 2c, the macrocycle adopts a less planar conformation. The CuII ion coordinates tridentately to the three interior triazine N atoms and the coordination environment is completed by the two Br− anions. The corresponding CuI complex 4c was synthesized in an analogous way and the solid-state structure shows very similar features (see ESI†).
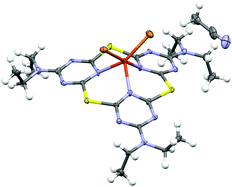 | ||
| Fig. 2 ORTEP representation of CuII complex 3c. Thermal displacement ellipsoids are shown at the 50% probability level. | ||
To gain more insight on the complex formation in solution UV/Vis titrations were performed (in MeCN). Calix[3]arene 2c exhibits a main broad absorption band centered at 259 nm (ε = 3.15 × 104 M−1 cm−1), while upon titration with CuIIBr2 a bathochromic shift to 273 nm and a concurrent increase in absorbance (ε = 6.17 × 104 M−1 cm−1) was observed (see ESI†). A clear isosbestic point could be seen at λ = 262 nm, indicating an equilibrium between the free ligand and its CuII complex. Titration curves were fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry model, as determined via single-crystal X-ray analysis, using HypSpec software and an association constant Kass ≥ 106 M−1 was obtained (Table 1).9Titration of 2c with CuIBr gave an almost identical shift and increase in intensity (ε = 6.72 × 104 M−1 cm−1). However, more equivalents of the Cu salt were required and therefore a lower affinity (Kass = 2.4 × 104 M−1) was derived (Table 1) (see ESI†).
1 stoichiometry model, as determined via single-crystal X-ray analysis, using HypSpec software and an association constant Kass ≥ 106 M−1 was obtained (Table 1).9Titration of 2c with CuIBr gave an almost identical shift and increase in intensity (ε = 6.72 × 104 M−1 cm−1). However, more equivalents of the Cu salt were required and therefore a lower affinity (Kass = 2.4 × 104 M−1) was derived (Table 1) (see ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation between hosts 2a–c and metal salts or anionic guests in dry MeCNa
1 complexation between hosts 2a–c and metal salts or anionic guests in dry MeCNa
| CuBr2 | CuBr | HSO4− | H2PO4− | HCO3− | AcO − | Cl − | |
|---|---|---|---|---|---|---|---|
| a Calculated on the basis of UV/Vis titration data using HypSpec.9b Values are the average of at least three separate λ's and are considered reproducible to 10%. b This value is ≥106 M−1. As such the stability constant is at the upper limit of what can be determined by this technique and should be treated with caution. c No notable spectral changes. | |||||||
| 2a | — | — | 0c | 30 | 545 | 3260 | 0c |
| 2b | — | — | 0c | 950 | 5240 | 8.6 × 104 | 0c |
| 2c | ≥106b | 2.4 × 104 | ≥106b | 1.2 × 104 | 0c | 0c | 0c |
Anion recognition by artificial macrocyclic receptors has attracted a lot of attention due to the increased awareness of the vital importance of anions in many biological and environmental systems.10 In this respect there is also a rising interest in the supramolecular exploitation of anion–arene interactions, caused by favorable interaction between anions and neutral electron poor (het)arenes.11 Recent studies have indicated that the π–acidic cavity of tetraoxacalix[2]arene[2]triazines is capable of halide anion binding, both in solution and in the solid state, due to a synergic effect of noncovalent halide–π and lone pair–π interactions.3d,h,j As we envisioned that the π–electron deficient cavity of the selenacalix[3]triazines could also be capable of anion recognition, a preliminary investigation towards their affinity for a small variety of putative anionic guests was performed. UV/Vis titrations of host 2c with Cl−, AcO− or HCO3− in MeCN did not induce any notable spectral changes. However, when hydrogen sulfate was titrated with the host solution, a clear isosbestic point and a noticeable bathochromic and hyperchromic shift were observed (Fig. 3). The Job plot revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (host
1 (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) stoichiometry (see ESI†) and an association constant Kass ≥ 106 M−1 was derived, pointing out the particularly high affinity of 2c for HSO4− (Table 1). The complex could also be identified by ESI-MS (see ESI†). In addition, a weaker interaction with the less acidic H2PO4− was also observed (Table 1). The type of complex and the preferred binding motif (anion–areneversus protonation) remain unknown, as solid-state evidence could not be obtained. Additional UV/Vis titrations for selenacalix[3]arenes 2a and 2b did not provide any firm clarification regarding this issue, as a reverse trend could be distinguished (AcO− > HCO3− > H2PO4−), while no interaction with HSO4− was observed (Table 1). The complexation is obviously very sensitive to the substitution pattern, which suggests two competitive mechanisms.7
guest) stoichiometry (see ESI†) and an association constant Kass ≥ 106 M−1 was derived, pointing out the particularly high affinity of 2c for HSO4− (Table 1). The complex could also be identified by ESI-MS (see ESI†). In addition, a weaker interaction with the less acidic H2PO4− was also observed (Table 1). The type of complex and the preferred binding motif (anion–areneversus protonation) remain unknown, as solid-state evidence could not be obtained. Additional UV/Vis titrations for selenacalix[3]arenes 2a and 2b did not provide any firm clarification regarding this issue, as a reverse trend could be distinguished (AcO− > HCO3− > H2PO4−), while no interaction with HSO4− was observed (Table 1). The complexation is obviously very sensitive to the substitution pattern, which suggests two competitive mechanisms.7
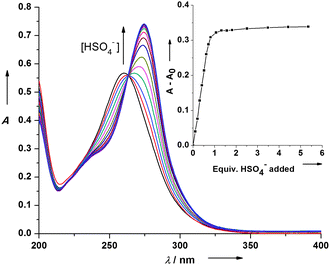 | ||
| Fig. 3 UV/Vis titration of 2c with (n-Bu)4N(HSO4) in MeCN. Inset: variation of absorbance at λ = 275 nm vs. equiv. of HSO4− added. | ||
In summary, selenacalix[3]triazines were synthesized selectively in a high yield, representing the first examples of Se-bridged heteracalixarenes and thereby enlarging the scope and supramolecular applications of heteroatom-bridged calix(hetero)aromatics. UV/Vis titration studies indicated high binding affinities for the complexation of Cu salts and anionophoric properties depending on the substitution pattern. Further elaboration of the synthetic and supramolecular chemistry of selenacalixarenes is currently actively being pursued within our group.
We thank the FWO, the KU Leuven and the Ministerie voor Wetenschapsbeleid for continuing financial support.
Notes and references
- Heteracalixarene reviews: (a) B. König and M. H. Fonseca, Eur. J. Inorg. Chem., 2000, 2303 CrossRef; (b) P. Lhotak, Eur. J. Org. Chem., 2004, 1675 CrossRef CAS; (c) N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291 CrossRef CAS; (d) H. Tsue, K. Ishibashi and R. Tamura, in Heterocyclic Supramolecules I, Topics in Heterocyclic Chemistry, ed. K. Matsumoto, Springer, Heidelberg, 2008, vol. 17, pp. 73–96 Search PubMed; (e) W. Maes and W. Dehaen, Chem. Soc. Rev., 2008, 37, 2393 RSC; (f) M.-X. Wang, Chem. Commun., 2008, 4541 RSC; (g) M.-X. Wang, Acc. Chem. Res., 2011 DOI:10.1021/ar200108c.
- Azacalixarenes: (a) Y. Miyazaki, T. Kanbara and T. Yamamoto, Tetrahedron Lett., 2002, 43, 7945 CrossRef CAS; (b) M.-X. Wang, X.-H. Zhang and Q.-Y. Zheng, Angew. Chem., Int. Ed., 2004, 43, 838 CrossRef CAS; (c) H.-Y. Gong, Q.-Y. Zheng, X.-H. Zhang, D.-X. Wang and M.-X. Wang, Org. Lett., 2006, 8, 4895 CrossRef CAS; (d) H. Tsue, K. Ishibashi, S. Tokita, H. Takahashi, K. Matsui and R. Tamura, Chem.–Eur. J., 2008, 14, 6125 CrossRef CAS; (e) B. Yao, D.-X. Wang, Z.-T. Huang and M.-X. Wang, Chem. Commun., 2009, 2899 RSC; (f) E.-X. Zhang, D.-X. Wang, Z.-T. Huang and M.-X. Wang, J. Org. Chem., 2009, 74, 8595 CrossRef CAS.
- Oxacalixarenes: (a) M.-X. Wang and H.-B. Yang, J. Am. Chem. Soc., 2004, 126, 15412 CrossRef CAS; (b) J. L. Katz, M. B. Feldman and R. R. Conry, Org. Lett., 2005, 7, 91 CrossRef CAS; (c) W. Maes, W. Van Rossom, K. Van Hecke, L. Van Meervelt and W. Dehaen, Org. Lett., 2006, 8, 4161 CrossRef CAS; (d) D.-X. Wang, Q.-Y. Zheng, Q.-Q. Wang and M.-X. Wang, Angew. Chem., Int. Ed., 2008, 47, 7485 CrossRef CAS; (e) W. Van Rossom, W. Maes, L. Kishore, M. Ovaere, L. Van Meervelt and W. Dehaen, Org. Lett., 2008, 10, 585 CrossRef CAS; (f) M.-L. Ma, X.-Y. Li and K. Wen, J. Am. Chem. Soc., 2009, 131, 8338 CrossRef CAS; (g) W. Van Rossom, M. Ovaere, L. Van Meervelt, W. Dehaen and W. Maes, Org. Lett., 2009, 11, 1681 CrossRef CAS; (h) D.-X. Wang, Q.-Q. Wang, Y. Han, Y. Wang, Z.-T. Huang and M.-X. Wang, Chem.–Eur. J., 2010, 16, 13053 CrossRef CAS; (i) S.-Z. Hu and C.-F. Chen, Chem. Commun., 2010, 46, 4199 RSC; (j) M. E. Alberto, G. Mazzone, N. Russo and E. Sicilia, Chem. Commun., 2010, 46, 5894 RSC; (k) W. Van Rossom, K. Robeyns, M. Ovaere, L. Van Meervelt, W. Dehaen and W. Maes, Org. Lett., 2011, 13, 126 CrossRef CAS.
- (a) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS; (b) G. Roy and G. Mugesh, J. Am. Chem. Soc., 2005, 127, 15207 CrossRef CAS; (c) J. A. Mukherjee, S. S. Zade, H. B. Singh and R. B. Sunoj, Chem. Rev., 2010, 110, 4357 CrossRef.
- (a) T. G. Back, Z. Moussa and M. Parvez, Angew. Chem., Int. Ed., 2004, 43, 1268 CrossRef CAS; (b) H. Xu, J. Gao, Y. Wang, Z. Wang, M. Smet, W. Dehaen and X. Zhang, Chem. Commun., 2006, 796 RSC; (c) K. P. Bhabak and G. Mugesh, Acc. Chem. Res., 2010, 43, 1408 CrossRef CAS.
- (a) G. Hua, Y. Li, A. M. Z. Slawin and J. D. Woollins, Angew. Chem., Int. Ed., 2008, 47, 2857 CrossRef CAS; (b) W. Levason, J. M. Manning, G. Reid, M. Tuggey and M. Webster, Dalton Trans., 2009, 4569 RSC; (c) A. Lari, R. Gleiter and F. Rominger, Eur. J. Org. Chem., 2009, 2267 CrossRef CAS; (d) A. Panda, Coord. Chem. Rev., 2009, 253, 1056 CrossRef CAS; (e) J. Thomas, W. Maes, K. Robeyns, M. Ovaere, L. Van Meervelt, M. Smet and W. Dehaen, Org. Lett., 2009, 11, 3040 CrossRef CAS.
- An upcoming study will focus on the synthetic details and elaborate on the supramolecular and solid-state features of these materials.
- Heteracalix[3]arenes: (a) M. Mascal, J. L. Richardson, J. A. Blake and W. Li, Tetrahedron Lett., 1997, 38, 7639 CrossRef CAS; (b) A. Ito, Y. Ono and K. Tanaka, J. Org. Chem., 1999, 64, 8236 CrossRef CAS; (c) R. Tanaka, T. Yano, T. Nishioka, K. Nakajo, B. K. Breedlove, K. Kimura, I. Kinoshita and K. Isobe, Chem. Commun., 2002, 1686 RSC; (d) Y. Suzuki, T. Yanagi, T. Kanbara and T. Yamamoto, Synlett, 2005, 263 CAS; (e) Y. Tsukahara, M. Hirotsu, S. Hattori, Y. Usuki and I. Kinoshita, Chem. Lett., 2008, 37, 452 CrossRef CAS; (f) N. Uchida, A. Taketoshi, J. Kuwabara, T. Yamamoto, Y. Inoue, Y. Watanabe and T. Kanbara, Org. Lett., 2010, 12, 5242 CrossRef CAS.
- (a) P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739 CrossRef CAS; (b) HypSpec software, Protonic Software, http://www.hyperquad.co.uk.
- J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, RSC, Cambridge, 2006 Search PubMed.
- (a) P. Gamez, T. J. Mooibroek, S. J. Teat and J. Reedijk, Acc. Chem. Res., 2007, 40, 435 CrossRef CAS; (b) B. P. Hay and V. S. Bryantsev, Chem. Commun., 2008, 2417 RSC; (c) B. L. Schottel, H. T. Chifotides and K. R. Dunbar, Chem. Soc. Rev., 2008, 37, 68 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization data, 1H and 13C NMR spectra, X-ray crystallographic data for 2c, 3c, and 4c, and additional details on the metal salt and anion titrations. CCDC 808717, 808718, 816095. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1cc15473g |
| This journal is © The Royal Society of Chemistry 2012 |
