Anisotropic two-dimensional sheets assembled from rod-shaped metal complexes†
Mina
Han
* and
Tomohiro
Hirade
Department of Chemistry and Department of Electronic Chemistry, Tokyo Institute of Technology, Yokohama 226-8502, Japan. E-mail: han.m.ab@m.titech.ac.jp; Fax: +81 45 924 5447; Tel: +81 45 924 5447
First published on 4th November 2011
Abstract
The facile formation of anisotropic two-dimensional sheets with different sizes, ranging from nanometre-scale to micrometre-scale, was achieved by the assembly of rod-shaped palladium complexes.
Supramolecular architectures with well-defined structures and desired functions have received a great deal of attention, because of their intrinsic optoelectronic properties and their potential applications.1,2 The formation of two-dimensional (2D) self-assembled nanostructures based on functional metal complexes and organic molecules is driven by various non-covalent interactions including π–π stacking, van der Waals, hydrogen bonding, surfactant effect, charge transfer, metal coordination, hydrophobic interactions, etc.1–3 Although Shelnutt et al.4 and Che et al.5 have succeeded in fabricating free-standing, planar nanosheets from Sn(IV)-containing porphyrin and square-planar Pt(II) complexes, respectively, using a simple reprecipitation method, facile synthetic procedures for controlling the size and shape of 2D nanostructures of functional metal complexes remain challenging.
Several investigations have been reported on mononuclear palladium complexes with azobenzene ligands.6 Nevertheless, it is difficult to prove the reversible photoswitching ability given by photoisomerizable azobenzene chromophores, owing to the facile dissociation of azobenzene ligands from palladium complexes. To overcome the drawback, we recently designed a new type of palladium complex containing ortho-alkylated azobenzene ligands, and verified reversible changes in the molecular conformation in response to different wavelengths of light.7 For developing a new class of photochemically and electrochemically responsive materials, the creation of free-standing, 2D nano- and microstructures from transition metal complexes is desirable.
Here we describe the formation of free-standing anisotropic sheets of azobenzene–palladium complexes (Scheme 1) using a simple reprecipitation method. Atomic force microscopy (AFM), transmission electron microscopy (TEM), optical microscopy (OM) images substantiate that the size of unique parallelogram-shaped aggregates was readily controlled from nanometre-scale to micrometre-scale by the variation in the initial concentration. Not only unidirectional molecular orientation of metal complexes but also the growth process of 2D sheet was examined.
 | ||
| Scheme 1 Chemical structure of PdCl2Az2. | ||
To prepare nano- and microstructured aggregates, we added water dropwise into PdCl2Az2 THF solution (1 × 10−4, 2 × 10−4, 2 × 10−3, and 8 × 10−3 M) under gentle stirring. With increasing water content, THF/water mixed solutions became orange milky and turbid.
The aggregation behavior of PdCl2Az2 could be observed by taking aliquots from each sample and characterizing the size and shape of the aggregates by AFM, TEM, OM. Suspensions in 2 × 10−5 M THF/H2O (1/4, v/v) contained unique parallelogram-shaped aggregates with an angle and area of 59–68° and <1.1 μm2, respectively (Fig. 1). Nanometre-scale aggregates were well dispersed at ambient temperature. The absorption spectrum indicates that a strong π–π* absorption band is slightly red-shifted to 355 nm with respect to the monomer-like absorption spectrum possessing λmax = 352 nm (in THF in Fig. 2c), together with a weak shoulder in the 350–390 nm range (see ESI†, Fig. S1). The spectral feature is likely associated with aggregation of coordinated azobenzene ligands in head-to-tail fashion, as inferred from the X-ray crystal structure.7
 | ||
| Fig. 1 Characterization of PdCl2Az2 parallelogram-shaped sheets. (a) and (b) 2 × 10−5 M THF/H2O (1/4, v/v). (c) and (d) 1 × 10−4 M THF/H2O (1/1, v/v). (e) and (f) 1.1 × 10−3 M THF/H2O (5/4, v/v). (g) 5.3 × 10−3 M THF/H2O (2/1, v/v). Images were obtained by TEM (a, c), AFM (inset in b) and OM (inset in d, e, g). The lateral dimensions (areas in b, d and f) and angles (in h) were calculated from TEM and OM images. | ||
 | ||
| Fig. 2 (a) POM and (b) OM images of microsheets. (c) Polarized UV-vis absorption spectral changes of a single microsheet (indicated by a yellow square in (b) as a function of polarization angle (α) of the probe light. | ||
By contrast, suspensions prepared from higher concentrations precipitated from the solvent upon short-term storage, but was re-dispersed by gentle shaking. Such a concentration dependence of precipitate behavior suggests the creation of different size or shape of aggregates. Obviously, Fig. 1a–g display that the size of unique parallelogram-shaped aggregates varies from tens of nanometres to tens of micrometres, depending on the initial concentration of the palladium complex. On the other hand, regardless of the lateral dimensions, the thickness is rather uniform in the range of 30–70 nm (see ESI†, Fig. S2), confirming the formation of 2D planar sheets. By comparison, the aggregation behavior of PdCl2Az2 was also investigated under different solvent conditions. The shape of aggregates depended on the solvent, and cuboid crystals (rather than planar sheets) were commonly seen from DMF/H2O solution (see ESI†, Fig. S3).
For a dilute THF solution, more water is needed to give an orange turbid suspension. Thus, a large amount of nuclei may be preferably generated in an early stage, which facilitates the formation of nanometre-scale aggregates. In contrast, for concentrated THF solutions, a relatively small ratio of water to THF solution is enough to fabricate micrometre-scale planar sheets, meaning that the growth of nuclei generated in an initial stage proceeds even faster than the generation of additional nuclei.
Long and thin sheets were frequently observed from a 1 × 10−4 M THF/H2O (1/1, v/v) sample (Fig. 1c and d and Fig. S2b, ESI†), and were occasionally broken and/or deformed during sample preparation. Interestingly, suspensions in 1.1 × 10−3 M THF/H2O (5/4, v/v) contained fairly uniform 2D sheets, with the length and width of 20–30 μm and ∼10 μm, respectively (Fig. 1e and f and Fig. S4, ESI†). The observed angle is found to be 59–63°, similar to that of nanometre-scale aggregates (Fig. 1h). Importantly, polarized optical microscopy (POM) images acquired under the crossed Nicol conditions (Fig. 2a and Fig. S5, ESI†) exhibit strong birefringence, which is explained in terms of in-plane (unidirectional) orientation of rod-shaped palladium complexes.8
To further corroborate unidirectional molecular orientation of PdCl2Az2 in parallelogram-shaped sheets, polarized absorption measurements were made on a single microsheet (indicated by a yellow square in Fig. 2b) using JASCO MSV-350 UV-vis microspectrophotometer. Based on the absorption spectra of the azobenzene ligand and PdCl2Az2, a conspicuous absorption band near 470 nm can be ascribed to an n–π* transition involving the azobenzene ligand and charge-transfer transition involving metal–ligand interactions.6,7 The band in the range of 320–400 nm can be assigned to azobenzene-based π–π* transition with the transition dipole moment parallel to the long axis of azobenzene. As shown in Fig. 2c, noticeable differences in the absorption intensity were detected, depending on the polarization of probe light; in sharp contrast to that measured at the polarization angle (α = 155°), the intense absorption band in the range of 300–600 nm was recorded in the spectrum taken at 65° parallel to the shorter side of the parallelogram-shaped sheet. Thus, the dichroic ratio (DR = A65°/A155°) is calculated to be 3.5 at 470 nm, strongly indicating that rod-shaped PdCl2Az2 molecules having two azobenzene ligands are oriented parallel to α = 65°, as illustrated in the inset of Fig. 2c.
In addition to the above polarized absorption spectra, X-ray diffraction (XRD) patterns of planar sheets were measured in reflection mode with CuKα radiation on a Bruker D8 diffractometer. The XRD pattern taken at 75° from the surface normal is comparable to the simulated pattern of the single crystal, but considerably different from that taken at 0° (see ESI†, Fig. S6). Even though the NMR data taken before and after the formation of complex sheets were identical to each other (Fig. S7, ESI†), apparent and new peaks at 2θ = 8.37, 16.70, 23.30° (corresponding to 10.6, 5.3, 3.8 Å, respectively) emerged in the XRD patterns. These peaks may be attributable to the molecular height (∼1.1 nm) and π–π staking (0.38 nm) between the aromatic rings, which is likely responsible for the formation of highly crystalline sheets through anisotropic growth.
To get insight into the growth process of the anisotropic 2D sheet, we also performed detailed AFM measurements. AFM topographic images acquired for parallelogram-shaped sheets revealed fairly smooth and flat surfaces (Fig. 3a and Fig. S2, ESI†), irrespective of their size. It is evident that the smallest clusters with the height of 1.1 ± 0.1 nm, corresponding to monolayer thickness, were found on the surface of complex sheets (Fig. 3(I)). In addition, the clusters of 3.2 ± 0.1 and 2.2 ± 0.2 nm in height, which are roughly three times and twice the molecular height, respectively, were observable as well (Fig. 3(II)). In combination with the XRD and polarized absorption spectral data, we can define the growth process of the PdCl2Az2 2D sheet as follows. With increasing the content of water in solution, rod-shaped PdCl2Az2 molecules interact with one another through π–π stacking interactions to generate nuclei. The small clusters nucleated along a given direction (parallel to the shorter side of the sheet in Fig. 2c) fuse with each other to generate wider 2D sheets (Fig. 3b). Neighboring complexes are stacked up with the molecular height of ∼1.1 nm, which reduces steric hindrance. As a consequence, self-assembled nano- and microstructures possess unidirectional molecular orientation of rod-shaped complexes, resulting in the obvious polarization dependence of absorption spectra and strong birefringence in POM images.
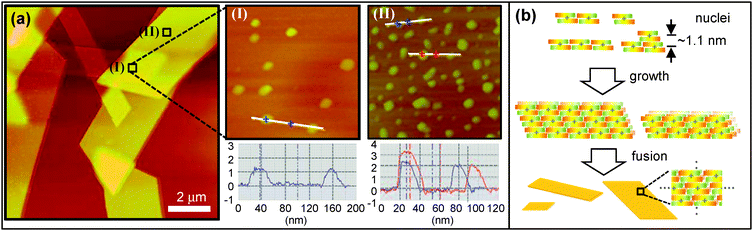 | ||
| Fig. 3 (a) AFM image of complex microsheets on a mica substrate. (I) and (II) correspond to zoom-in images and height profiles. (b) A schematic representation of the proposed growth process. | ||
In summary, we have demonstrated the formation of free-standing anisotropic 2D sheets of rod-shaped azobenzene–palladium complexes using a simple reprecipitation method. The size of parallelogram-shaped structures, ranging from nanometre-scale to micrometre-scale, can be readily adjusted by changing the complex concentration. In-plane molecular orientation of metal complexes through π–π stacking interaction and uniform intermolecular distance is responsible for strong birefringence and dichroism of microsheets. Such anisotropic sheets consisting of photoresponsive metal complexes might be interesting materials for applications in molecular electronics and photonics.
This work was supported by the Global COE program from the Ministry of Education, Culture, Sports, Science, and Technology of Japanese Government. We are grateful to Prof. T. Iyoda and K. Matsumoto for TEM measurements, as well as Prof. T. Ikeda, Prof. A. Shishido and Dr N. Ishii for POM measurements. We also wish to thank Center for Advanced Materials Analysis (Ookayama campus), Technical Department, Tokyo Institute of Technology, for XRD experiments.
Notes and references
- (a) J.-M. Lehn, Supramolecular Chemistry, VCH Press, New York, 1995 Search PubMed; (b) L. F. Lindoy and I. M. Atkinson, in Self-assembly in Supramolecular Systems, ed. J. F. Stoddart, Royal Society of Chemistry, Cambridge, UK, 2000 Search PubMed.
- (a) G. M. Whitesides, J. P. Mathias and C. T. Seto, Science, 1991, 254, 1312–1319 CAS; (b) S. I. Stupp, V. LeBonheur, K. Walker, L. S. Li, K. E. Huggins, M. Keser and A. Amstutz, Science, 1997, 276, 384–389 CrossRef CAS; (c) D. Horn and J. Rieger, Angew. Chem., Int. Ed., 2001, 40, 4330–4361 CrossRef CAS.
- (a) H. Maeda, Y. Kusunose, M. Terasaki, Y. Ito, C. Fujimoto, R. Fujii and T. Nakanishi, Chem.–Asian J., 2007, 2, 350–357 CrossRef CAS; (b) R. Davis, R. Berger and R. Zentel, Adv. Mater., 2007, 19, 3878–3881 CrossRef CAS; (c) M. Sathish and K. Miyazawa, J. Am. Chem. Soc., 2007, 129, 13816–13817 CrossRef CAS; (d) L. Wang, B. Liu, D. Liu, M. Yao, S. Yu, Y. Hou, B. Zou, T. Cui, G. Zou, B. Sundqvist, Z. Luo, H. Li, Y. Li, J. Liu, S. Chen, G. Wang and Y. Liu, Appl. Phys. Lett., 2007, 91, 103112–103113 CrossRef; (e) X. Zhang, X. Zhang, B. Wang, C. Zhang, J. C. Chang, C. S. Lee and S. T. Lee, J. Phys. Chem. C, 2008, 112, 16264–16268 CrossRef CAS; (f) T. Wakahara, M. Sathish, K. Miyazawa, C. P. Hu, Y. Tateyama, Y. Nemoto, T. Sasaki and O. Ito, J. Am. Chem. Soc., 2009, 131, 9940–9944 CrossRef CAS; (g) E. Lee, J.-K. Kim and M. Lee, Angew. Chem., Int. Ed., 2009, 48, 3657–3660 CrossRef CAS; (h) Y. Lei, Q. Liao, H. Fu and J. Yao, J. Phys. Chem. C, 2009, 113, 10038–10043 CrossRef CAS; (i) Q. An, Q. Chen, W. Zhu, Y. Li, C. A. Tao, H. Yang, Z. Li, L. Wan, H. Tian and G. Li, Chem. Commun., 2010, 46, 725–727 RSC.
- Z. Wang, Z. Li, C. J. Medforth and J. A. Shelnutt, J. Am. Chem. Soc., 2007, 129, 2440–2441 CrossRef CAS.
- Y. Chen, K. Li, W. Lu, S. S. Y. Chui, C. W. Ma and C. M. Che, Angew. Chem., Int. Ed., 2009, 48, 9909–9913 CrossRef CAS.
- (a) J. F. Vanbaar, K. Vrieze and D. J. Stufkens, J. Organomet. Chem., 1974, 81, 247–259 CrossRef CAS; (b) G. P. Khare, R. G. Little, J. T. Veal and R. J. Doedens, Inorg. Chem., 1975, 14, 2475–2479 CrossRef CAS; (c) L. P. Wu, Y. Suenaga, T. Kuroda-Sowa, M. Maekawa, K. Furuichi and M. Munakata, Inorg. Chim. Acta, 1996, 248, 147–152 CrossRef CAS; (d) S. Park, O. N. Kadkin, J. G. Tae and M. G. Choi, Inorg. Chim. Acta, 2008, 361, 3063–3068 CrossRef CAS.
- M. Han, T. Hirade and M. Hara, New J. Chem., 2010, 34, 2887–2891 RSC.
- (a) M. Han, S. Morino and K. Ichimura, Macromolecules, 2000, 33, 6360–6371 CrossRef CAS; (b) A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139–4175 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis absorption spectrum, AFM images and height profiles, TEM image of cuboid crystals, POM and OM images and XRD patterns. See DOI: 10.1039/c1cc14937g |
| This journal is © The Royal Society of Chemistry 2012 |
