Rhodamine and BODIPY chemodosimeters and chemosensors for the detection of Hg2+, based on fluorescence enhancement effects
M. J.
Culzoni
*a,
A.
Muñoz de la Peña
*b,
A.
Machuca
b,
H. C.
Goicoechea
a and
R.
Babiano
c
aLaboratorio de Desarrollo Analítico y Quimiometría (LADAQ), Cátedra de Química Analítica I, Facultad de Bioquímica y Ciencias Biológicas, Universidad Nacional de Litoral, Santa Fe, S3000ZAA, Argentina
bDepartment of Analytical Chemistry, University of Extremadura, 06006, Badajoz, Spain
cDepartment of Organic and Inorganic Chemistry, University of Extremadura, 06006, Badajoz, Spain
First published on 6th November 2012
Abstract
Fluorescent sensors for Hg2+ are demonstrating their potential in a variety of fields such as environmental and biological applications. The review focuses on the recent development of rhodamine derivatives in which the spirolactam (non-fluorescent) to ring-opened amide (fluorescent) process was utilized and on the development of BODIPY derivatives in which the photoinduced electron transfer (PET) process was utilized. New trends in the immobilization of the molecular probes on solid supports, as polymers and/or nanostructures, have been emphasized. The different recognition mechanisms used for the signal responses have been analyzed. The spectroscopic properties, reaction media, analytical parameters, interferences by other ions and practical applications have been summarized.
 M.J. Culzoni | María Julia Culzoni was born in Rafaela, Santa Fe, Argentina, in 1979. She received her PhD from Universidad Nacional del Litoral (2008). After completing postdoctoral research at Universidad Nacional de Rosario, Argentina, she joined the faculty at Universidad Nacional del Litoral in 2010, where she is currently a teaching assistant of Analytical Chemistry and Chemometrics. Since 2010, she has been working as a researcher at the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. Her research interests include the development of sensors and analytical methods based on fluorescence spectroscopy and separations coupled to efficient extraction of information using chemometric modeling. |
 A. Muñoz de la Peña | Arsenio Muñoz de la Peña received his PhD (1981) degree at the University of Extremadura, Badajoz, Spain. He held post-doctoral positions at the Department of Medicinal Chemistry, University of Florida, and Department of Chemistry, University of Emory, and maintained a long-term collaboration with the Universities of Rosario and Litoral (Argentina). He is full Professor and Head of the Department of Analytical Chemistry at the University of Extremadura. He is author of more than 170 scientific articles, reviews and book chapters. His research interest includes analytical applications of luminescence, multi-way multivariate calibration, and the development of luminescence chemosensors. |
 A. Machuca | Alejandro Machuca was born in Badajoz, Spain, in 1988. He received his B.Sc. (2011) and M.Sc. (2012) from the University of Extremadura, and is performing pre-doctoral research at the Department of Analytical Chemistry at the University of Extremadura. His research interest is related to the development of luminescence chemosensors and chemodosimeters for environmental analysis. |
 H. C. Goicoechea | Héctor Goicoechea was born in Santa Fe, Argentina, in 1961. He received his PhD (2000) from the National University of Rosario. After completing postdoctoral research at Department of Chemistry and Molecular Biology, North Dakota State University, Fargo, USA, he joined the faculty at National University of Litoral, Santa Fe, in 2004, where he is currently a Full Professor of Analytical Chemistry and Chemometrics. He belongs to the National Council of Scientific and Technical Research (CONICET) of Argentina. His research interests include development of analytical methods based on fluorescence spectroscopy and separations coupled to chemometric modelling. |
 R. Babiano | Reyes Babiano received his PhD degree in Chemistry from the University of Extremadura, where he is currently Associate Professor of Organic Chemistry. He has published over 100 papers in peer-reviewed journals. His research interests focus on different domains of basic and applied chemistry, such as development of asymmetric transformations with chiral auxiliaries, practical and theoretical aspects of chirality, molecular recognition, gelation phenomena, chemical sensors, and improved materials via surface modification. |
1 Introduction
There is great interest in the development of good sensors for the detection of heavy metal ions because, although some have vital and beneficial effects, the toxicity of others is of particular concern. It is a fact that Hg2+, Cd2+, Pb2+ and As3+ are among the most toxic ions known that lack any vital or beneficial effects and that accumulation of these over time in the bodies of humans and animals can lead to serious illnesses. Specifically, Hg2+ is considered as one of the most hazardous environmental contaminants.1–3 It is widely distributed in air, water, and soil4,5 through different processes such as volcanic emissions, mining, solid waste incineration, and the combustion of fossil fuels.6–8Of particular concern is the ability of some anaerobic organisms to transform the elemental and inorganic forms of mercury into methylmercury, allowing its entrance in the food chain through bioaccumulation in edible animals, and subsequent introduction in the human body.9–12 Besides, mercury ions can easily pass through biological membranes13 and show a high affinity for thiol groups in proteins.14,15
It is a considerably dangerous metal to human life and ecology even at low concentrations. Among the serious health consequences due to the presence of Hg2+ in the human body are prenatal brain damage, DNA damage, various cognitive and motion disorders, Minamata disease, myocardial infarction, some kinds of autism and damage of the brain, kidneys, central nervous system, immune system and endocrine system.13–17
The usual methods for determination of mercury are atomic absorption spectroscopy,18–20 inductively coupled plasma mass spectrometry (ICP-MS),21–23 capillary electrophoresis–ICP-MS24 and high performance liquid chromatography–ICP-MS.25–27
Taking into account the fact that these techniques demand expensive and very complicated sample pretreatment and instrumentation, the development of optical probes able to translate molecular recognition into tangible optical signals is highly demanded. Moreover, the synthesis and design of optical sensors are focused on the capability of detecting mercury at a low cost with high sensitivity and selectivity.9,28 Noticeably, there is a great variety of chemosensors and chemodosimeters capable of providing useful information about the appearance of mercury in different matrices.
In this context, fluorescent molecular sensors are becoming more and more important in mercury detection due to their easy use, low cost and high efficiency. Generally, Hg2+ ions are known to produce fluorescence quenching when binding to fluorophore molecules via the spin–orbit coupling effect. In consequence, the turn-off is the usual response upon binding in most instances, and the sensors with fluorescence enhancement (turn-on response) are still rare. However, fluorescent probes showing fluorescence enhancement on binding to Hg2+ are preferred rather than quenching for the design of a metal-ion selective fluorescent sensor, as due to the ubiquitous nature of fluorescence quenching, their sensitivity and practical utility are to some extent reduced.
Therefore, development of fluorescent turn-on type response molecular probes for monitoring the level of Hg2+ in environmental and biological samples is of current interest.29
2 Fluorescent chemodosimeters and chemosensors
Chemodosimeters are devices, molecule-sized or larger, based on receptors, to achieve analyte recognition with concomitant irreversible transduction of a human-observable signal.30 A chemodosimeter allows analyte detection through a highly selective and usually irreversible chemical reaction between the dosimeter molecule and the target analyte leading to an observable signal (some physical change), in which an accumulative effect is directly related to the analyte concentration.On the other hand, chemosensors are molecules that interact with the analyte to yield measurable signals with a real-time response (usually less than a few seconds). Chemosensors, contrarily to chemodosimeters, work in a general operating principle which is based on coordination events. Consequently, the reaction of a chemosensor with the target analyte and the accompanying signal changes are reversible, which is the main difference between chemosensors and chemodosimeters.31 It is important to remark that both kinds of sensors should ideally be selective for a particular guest and not only report the presence of the analyte, but should also allow monitoring its concentration. This is important medically (for monitoring indicators of physical functions) and environmentally (monitoring pollutant levels). Frequently, the constitution of sensors with adequate properties is only possible through the design of suitable abiotic receptors.
Fluorogenic chemodosimeters and chemosensors can report the presence of an analyte via changes in the measurable photophysical property of the system and, as stated above, both quenching and enhancement of fluorescence can be used to detect the presence of an analyte, although for practical reasons enhancement is preferable in sensors as the decreased emissions have low signal outputs upon interaction with the analyte of interest.
As was previously mentioned, chemodosimeters/chemosensors are potentially useful in chemical as well as biological systems. Especially, the use of sensor molecules for Hg2+ that present instantaneous measurable optical response is highly attractive.
Interestingly, sensitivity of fluorescence techniques is important in this context. While absorbance measurements for colorimetric sensors can reliably determine concentrations only as low as several tenths of a micromole, fluorescence techniques can accurately measure concentrations one million times smaller. Due to these advantages fluorescent sensors are especially attractive as they give a meaningful physical output which is easy to measure even at low concentrations. Thus, fluorescent chemosensor design is an active field of supramolecular chemistry, not only because of potential practical benefits in cell physiology and analytical and environmental chemistry, but also as a proving ground for manipulation and/or engineering of various photophysical processes toward an ultimate goal of selective and sensitive signaling of targeted molecular or ionic species.32,33
3 Rhodamine-based chemodosimeters and chemosensors for Hg2+
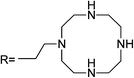
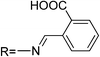
Rhodamine spirolactam based molecular probes have attracted a lot of attention since the pioneering work of Czarnik et al.30 Since then, a number of rhodamine derivatives have been synthesized as chemodosimeters and/or chemosensors for different metal ions including mercury. Recent reviews deal with the detection of Hg2+ and other ions with rhodamine based sensors.34,35A particularly attractive approach is the use of fluorimetric chemodosimeters for the determination of Hg2+, through a specific chemical reaction between dosimeter molecules and the target species. This involves the use of highly selective reactions that can be reversible or irreversible, induced by the presence of mercury ions, in which a fluorescence enhancement effect is directly related to analyte concentration. In Tables 1 and 2, the reported molecular probes, the spectroscopic and analytical parameters and practical applications of the proposed rhodamine 6G and B derivatives for Hg2+ determination are summarized.
|
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensor compound | Structure | ∅ | [R] (μM) | Reaction medium | λ exc (nm) | λ em (nm) | Linear range (ng mL−1) | LOD (ng mL−1) | Interferences | R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+ stoichiometry Hg2+ stoichiometry |
Reversibility | Analytical applications | Ref. |
| RG1 |

|
0.42 | 5 | DMF–H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), pH 5–10 50 v/v), pH 5–10 |
500 | 560 | 2–20 | 2 | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 36 |
| RG2 |
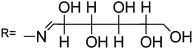
|
0.48 | 0.1 | H2O, pH 5–10 | 500 | 550 | 2–10 | 1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding Na2S | Living cells and natural waters (seawater, drinking water) | 37 |
| RG3 |

|
— | 10 | MetOH–H2O (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v), pH 5–10 (pH 7) 10 v/v), pH 5–10 (pH 7) |
520 | 555 | (2–100) × 103 | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Irreversible | — | 38 |
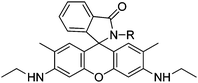
| RG4 |
|
0.52 | 0.1 | MetOH–H2O (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v), pH 4–14 (pH 7.4) 20 v/v), pH 4–14 (pH 7.4) |
515 | 557 | 1–12 | 0.7 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Irreversible | Living cells and zebra fish organs, water and fish samples | 39–46 |
| RG5 |

|
— | 0.1 | DMSO–MetOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 v/v), pH 5–10 90 v/v), pH 5–10 |
520 | 550 | 1–9 | 1 | Slight interference from Ag+, Zn2+, Cu2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Irreversible | Living cells | 47 |
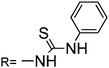
| RG6 |
|
0.38 | 0.1 | H2O, pH 5.5–12 (pH 7) | 500 | 550 | 1–5 | 1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding Na2S | Natural waters | 48 |
| RG7 |
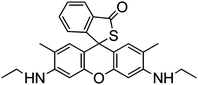
|
— | 10 | 515 | 545 | 0.2–2 | — | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding KI | In vivo analysis using C. elegans, adapted for portable LED based fluorimeters | 49 and 50 | |
| RG8 |
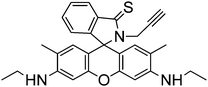
|
— | 5 | DMF–H2O, (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v), pH 6–7.4 (pH 7.2) 80 v/v), pH 6–7.4 (pH 7.2) |
500 | 566 | 10–800 | 8 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Partial reversibility | Living cells | 51 |
| RG9 |
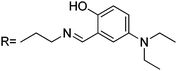
|
— | 5 | EtOH–H2O, (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v), pH 7 4 v/v), pH 7 |
500 | 556 | (0.1–2) × 103 | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 52 |
|
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensor compound | Structure | ∅ | [R] (μM) | Reaction medium | λ exc (nm) | λ em (nm) | Linear range (ng mL−1) | LOD (ng mL−1) | Interferences | R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+ stoichiometry Hg2+ stoichiometry |
Reversibility | Analytical applications | Ref. |
| RB1 |
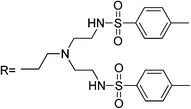
|
— | 1 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, (90 H2O, (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v), pH 3–6 10 v/v), pH 3–6 |
520 | 575 | (0.2–2) × 103 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 53 | |
| RB2 |
|
— | 6 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (95 H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) 5 v/v) |
554 | 575 | (0.2–12) × 103 | — | Cd2+,Zn2+, Pb2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Reversible | — | 54 |
| RB3 |
|
— | 6 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (95 H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) 5 v/v) |
554 | 575 | — | — | Zn2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Reversible | — | 54 |
| RB4 |
|
0.15 | 20 | EtOH–H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), pH 7.2 50 v/v), pH 7.2 |
520 | 580 | — | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding I− | Living cells | 55 |
| RB5 |

|
— | 5 | MeCN/HEPES, (15/85), pH 6–12 (pH 6.98) | 530 | 597 | — | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding EDTA | — | 56 |
| RB6 |
|
— | 10 | H2O, pH 5.5–12 (pH 7) | 490 | FRET ratio: 591/520 | 0–200 | 10 | Cr3+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Irreversible | — | 57 |
| RB7 |
|
0.19 | 5 | CH3CN | 530 | 580 | (3–13) × 103 | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Yes, by adding triethylene-tetramine | — | 58 |
| RB8 |

|
— | 10 | CH3CH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 v/v) 99 v/v) |
510 | 580 | 0.2–200 | 0.2 | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding KI | In vivo C. elegans | 59 |
| RB9 |
|
— | 10 | CH3CH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 v/v) 99 v/v) |
510 | 578 | 0.2–200 | 0.8 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding KI | In vivo C. elegans | 59 |
| RB10 |
|
0.51 | 10 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (50 H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) 50 v/v) |
520 | 575 | (0.4–4) × 103 | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
— | — | 60 |
| RB11 |
|
— | 10 | 1,4-Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, (1 H2O, (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99), pH 3.40 99), pH 3.40 |
530 | 582 | 80–600 | — | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding KI | — | 61 |
| RB12 |
|
— | 5 | H2O, pH 4–10 (pH 7.4) | 510 | 591 | — | — | Cu2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding Na2S | Living cells | 62 |
| RB13 |
|
— | 1 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (50 H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), pH 5.58–9.21 (pH 7.0) 50 v/v), pH 5.58–9.21 (pH 7.0) |
515 | 593 | 1–6 | 0.4 | Slight interference from Ag+ and Cu2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding Na2S | Living cells | 63 |
| RB14 |
|
— | 5 | 1-4Dioxane ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (0.5 H2O (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99.5 v/v), pH 3–11 99.5 v/v), pH 3–11 |
530 | 585 | (1–10) × 102 | 4 | Slight interference from Ag+ | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Depending on the Hg2+ concentration by adding KI | — | 64 |
| — | 10 | CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 v/v), pH 4 99 v/v), pH 4 |
530 | 585 | (4–40) × 103 | — | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Yes, by adding KI | — | 65 | ||
| RB15 |
|
0.75 | 5 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (50 H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), pH 7 50 v/v), pH 7 |
510 | 580 | 1–5 | 0.9 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Irreversible | Natural water samples and living cells | 66 |
3.1 Rhodamine 6G derivatives
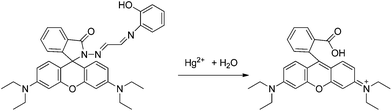
Most of the rhodamine-derived chemical sensors follow the approach of “spirolactam ring opening” for the detection of mercury ions, as the rhodamine derivatives with a spirolactam structure are non-fluorescent, whereas ring-opening of the spirolactam will give rise to a strong fluorescence emission.Usually, rhodamine system based sensors follow the mechanism shown in Scheme 1. This system is formed by the rhodamine fluorophore linked to a mercury receptor (dashed line rectangle) with different structures in each sensor. In the absence of the analyte, the sensor fluorophore has the spirolactamic structure, with a carbon atom with sp3 hybridization that prevents planarity and electronic delocalization between aromatic rings. After Hg2+ complexation in the receptor, a strong structural change is produced, which implies C–N spiranic bond rupture and formation of dideoxidiaminofluorone rings of rhodamines.
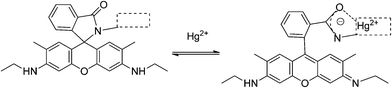 | ||
| Scheme 1 Spiranic system opening induced by Hg2+ complexation. | ||
Specificity and binding to the rhodamine derivatives depend on the type and spatial distribution of the donors attached and are frequently influenced by the nature of the solvents used.
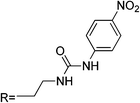
Among the rhodamine 6G derivatives, Wu et al. reported a highly sensitive fluorescence probe (sensor RG1) containing a carbohydrazone binding unit, selective for Hg2+ in mixed dimethylformamide (DMF) aqueous media,36 detecting ng mL−1 of Hg2+. The proposed mechanism of fluorescence enhancement of RG1 upon the addition of Hg2+ involves the formation of a ring-opened amide form from the initial spirolactam form (Scheme 2). No significant spectral changes of the sensor occur in the presence of a number of other ions, due to several combined influences cooperating to achieve the unique selectivity for Hg2+ ions, such as the suitable coordination geometry conformation of the bischelating Schiff-base receptor, the larger radius of the Hg2+ ion, and the nitrogen-affinity character of the Hg2+ ion. A 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry R
1 stoichiometry R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+ was proposed and the complexation mechanism is shown in Scheme 2. The proposed probe was not applied in real samples.
Hg2+ was proposed and the complexation mechanism is shown in Scheme 2. The proposed probe was not applied in real samples.
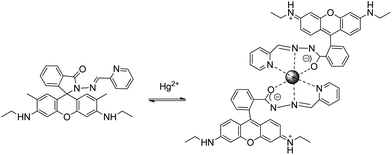 | ||
| Scheme 2 Proposed binding mode of RG1 with Hg2+. | ||
By combination of a water soluble sugar group and a rhodamine group in a molecule, a bright water compatible and specific sensor for Hg2+ (sensor RG2) in natural waters and living cells was achieved with a 1 ng mL−1 limit of detection, useful for monitoring Hg2+ within biological samples.37 Changes in the fluorescence band suggest that as a result from RG2–Hg2+ binding, a xanthene moiety is formed after the spiro ring is opened. The chemosensor was applied to Hg2+ determination in spiked natural water samples, seawater from Yellow Sea and freshwater from West Hill reservoir (Dalian, area of china), with satisfactory results. Also, the practical ability of RG2 as a Hg2+ probe was demonstrated in the fluorescence imaging of HeLa living cells.
Sensor RG3 was reported for Hg2+ chemosensing inducing a new 555 nm peak through the formation of a stable cyclic product by an irreversible desulfurization reaction,38 requiring more than 10 minutes to complete the reaction. No practical application was described. From the molecular structure, it is concluded that the addition of the Hg2+ ion induced the N atom of the spirolactam to attack the C atom of the thiourea, and thus a ring opening of the spirolactam rhodamine took place, followed by a removal of HgS and the formation of intramolecular guanylation (Scheme 3).
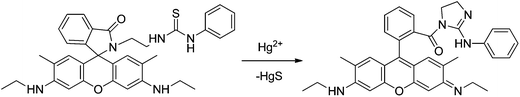 | ||
| Scheme 3 Proposed mechanism of Hg2+-induced ring opening and cyclization of RG3. | ||
Yang et al. first reported a fluorescent and colorimetric rhodamine-6G derivative (sensor RG4) that uses promoted desulfurization and cyclization reaction giving rise to 26-fold fluorescence enhancement and a high quantum yield of the reaction product.39 The RG4–Hg2+ interaction facilitates the spyrocycle ring opening and allows thiosemicarbazide to oxadiazole transformation (Scheme 4). The system was successfully applied to monitor exogenous Hg2+ uptake in living cells and vertebrate organisms in real time40,41 and also applied for methylmercury detection.42 Later on, it was applied to the determination of Hg2+ in waters and fish samples, the fluorescence intensity being proportional to the amount of Hg2+ at ng mL−1 levels, capable of distinguishing between safe and toxic levels of inorganic mercury. The procedure was implemented in a portable instrument composed of a 515 nm light-emitting diode (LED) excitation source, two filter optics, and a charge-coupled device (CCD) camera as detector, connected to a portable computer for data acquisition and analysis, intended for in situ determination of mercury, and offering a viable alternative to a conventional spectrofluorimeter.43
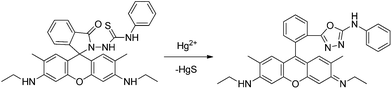 | ||
| Scheme 4 Proposed mechanism of Hg2+ induced ring opening and cyclization of RG4. | ||
This chemodosimeter has been also immobilized in nylon membranes and applied to the determination of Hg2+ in mineral, underground and river water samples, enhancing the sensitivity of the reaction with a LOD of 0.4 ng mL−1.44,45 Also, the probe has been immobilized in a polymeric membrane by a reverse atom transfer radical polymerization (ATRP) technique, and it is constituted by methylmethacrylate (MMA) and hydroxyethylmethacrylate (HEMA) units, as a preliminary step for the development of a new Hg2+-sensing film.46
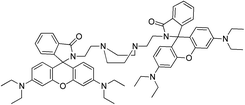
Xi et al. reported sensor RG5 with a remarkably high selectivity and a LOD of 2 ng mL−1 in dimethylsulfoxide (DMSO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol solutions47 that was applied to HeLa living cells to map its subcellular distribution, localizing the fluorescence signals in the perinuclear area of cytosol. The authors used a multichannel molecular system that incorporated two rhodamine fluorophores into a diisothiocyanate molecule to form a dual-rhodamine urea, instead of a common monorhodamine chemodosimeter system. Spectral changes in the emission band after Hg2+ addition can be attributed to delocalization in the xanthene moiety via spirocyclic opening.
methanol solutions47 that was applied to HeLa living cells to map its subcellular distribution, localizing the fluorescence signals in the perinuclear area of cytosol. The authors used a multichannel molecular system that incorporated two rhodamine fluorophores into a diisothiocyanate molecule to form a dual-rhodamine urea, instead of a common monorhodamine chemodosimeter system. Spectral changes in the emission band after Hg2+ addition can be attributed to delocalization in the xanthene moiety via spirocyclic opening.
A new chemosensor combining a ferrocene unit and a rhodamine 6G derivative block via the linkage of carbohydrazone binding units was prepared for the detection of Hg2+ in natural water with a LOD of 1 ng mL−1. The sensor–Hg2+ complex was isolated and it was demonstrated that the Hg2+ binding is reversible48 (sensor RG6), and needs previous spirocyclic opening (Scheme 5). The reported dye meets the criteria of appropriate selectivity over the competing metal ion contaminants and of optical sensitivity in an aqueous solution, and was applied to Hg2+ determination in spiked seawater from the Yellow Sea and freshwater from the West Hill Reservoir (Dalian, area of China).
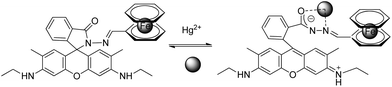 | ||
| Scheme 5 Proposed binding mode of RG6 with Hg2+. | ||
Chen et al. reported a rhodamine 6G thiolactone derivative sensor RG7 detecting Hg2+ in the nanomolar range in neutral aqueous solutions which was applied for in vivo imaging of Hg2+ using the bacteriovorous nematode C. elegans, which has been considered as an ideal organism for testing the toxicity of aquatic media such as municipal and industrial wastewater.49 Later on, the chemosensor was adapted to a light emitting diode (LED) exciting, fiber-optic and charged coupled device (CCD) based portable spectrofluorimeter, for analysis of environmental water samples.50 The large fluorescence enhancement is due to the induced spirothiolactone ring opening via Hg2+ complexation (Scheme 6).
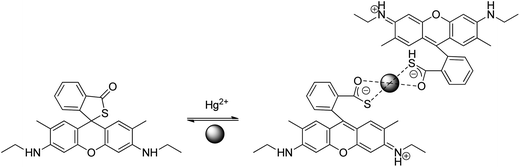 | ||
| Scheme 6 Proposed binding mode of RG7 with Hg2+. | ||
A new fluorescence turn-on probe (sensor RG8) for Hg2+ imaging in living cells was designed based on the interactions of Hg2+ with both thiol and alkyne moieties in a rhodamine 6G scaffold. The selection of thiol and alkyne moieties was based on the thiophilic and π-philic nature of Hg2+.51 The probe exhibits large fluorescence enhancement (140-fold), high selectivity, low detection limit and fast response, as the fluorescence enhancement reached the maximum within 3 minutes, and was satisfactorily applied for Hg2+ imaging in human Tca-8113 living cells. The proposed mechanism of the Hg2+-induced turn-on response is shown in Scheme 7, as concluded by mass-spectrometry and NMR analysis, thiozolcarbaldehyde being the minor product. The mechanism is consistent with the fact that when IK is added to the probe + Hg2+, only partial fluorescence quenching was noted, indicating that the sensing process is not completely reversible.
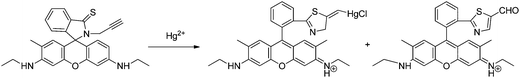 | ||
| Scheme 7 Proposed binding mode of RG8 with Hg2+. | ||
A new rhodamine 6G-derived Schiff base (sensor RG9) was reported as a chemosensor with good selectivity towards Hg2+, and a wide pH span (6–10), giving a good linear relationship in the concentration range from 0.5 to 10 μM, although the sensitivity was poor and it was not applied to real samples.52 From the molecular results and spectral changes observed, it was concluded that the addition of Hg2+ ions induced its complexation with carbonyl groups in spirolactam, N atoms in Schiff base, and hydroxyl in salicylaldehyde, as depicted in Scheme 8.
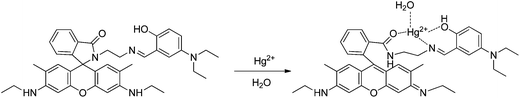 | ||
| Scheme 8 Proposed binding mode of RG9 with Hg2+. | ||
3.2 Rhodamine B derivatives
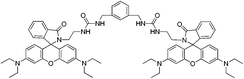
Lee et al. reported sensor RB1 as a N-tripodal structural compound consisting of a rhodamine B moiety and two branched tosyl group derivatives that is sensitive to Hg2+ in a CH3CN solution.53 The structure of the tripodaltris(2-aminoethyl)amine moiety (tren) has a good chelating ability for Hg2+ ions. The spirolactam-structured rhodamine moiety is directly linked to tren, and when the binding event takes place, it also leads to a ring-opening of spirolactam along with an obvious OFF–ON optical signal, and the strong electron-acceptor-two tosyl groups further produce a strong binding ability with Hg2+. Application to real samples was not reported.Two different rhodamine derivatives bearing the urea groups (sensors RB2–RB3) were reported by Soh et al.54 The sensor RB2 also exhibits reaction with Cd2+, Zn2+ and Pb2+, whereas sensor RB3 exhibits reaction with Zn2+. The sensitivity is poor in both cases and the dimmeric system displayed a higher selective fluorescent enhancement upon the addition of Hg2+. Complexation with metal ions forces ring opening to conjugated xantene moiety, and the two carbonyl oxygens provide a nice binding pocket for Hg2+ as shown in Scheme 9. Application of the probe to real samples was not reported.
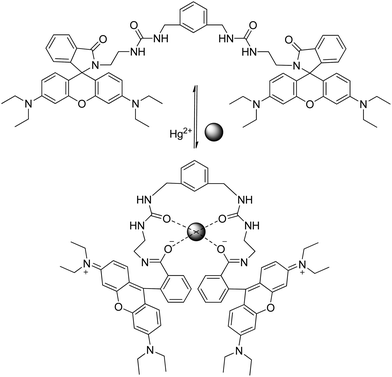 | ||
| Scheme 9 Proposed binding mode of RB3 with Hg2+. | ||
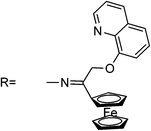
A new multisignaling optical–electrochemical sensor based on rhodamine B with a ferrocene subunit (sensor RB4) has been synthesized and has been shown to display extreme selectivity for Hg2+ over other metal ions. Multisignaling changes are observed through UV absorption, fluorescence emission, and electrochemical measurements, and applied for monitoring Hg2+ in living cells.55 The reaction is reversible (Scheme 10), which implies that RB4 acts as a chemosensor and not as a chemodosimeter for Hg2+. Laser confocal fluorescence microscopy was used for Hg2+ monitoring in Caor-3 ovarian carcinoma cells, showing that the fluorescence signals are localized in the perinuclear area of cytosol, indicating a subcellular distribution of Hg2+.
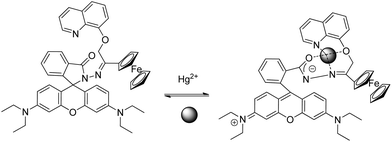 | ||
| Scheme 10 Proposed binding mode of RB4 with Hg2+. | ||
Huang et al. reported a rhodamine fluorophore (sensor RB5) by incorporation of ionophore NS2, which was established to be reversible by EDTA addition,56 as a result of the regeneration of the spirocyclic moiety (Scheme 11). The receptor contained the NS2 fragment, which is a well-known specific and reversible binding receptor of Hg2+ due to the thiophilic nature of Hg2+. Compared with other rhodamine-based chemosensors, RB5 reduced the amount of organic co-solvent in detecting media incorporating sensitivity, although application of the probe to real samples was not reported.
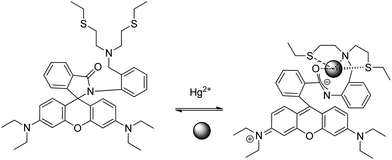 | ||
| Scheme 11 Proposed binding mode of RB5 with Hg2+. | ||
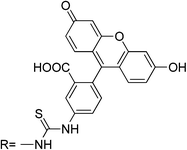
Based on an intramolecular fluorescence resonance energy transfer (RET) mechanism, Shang et al. reported a new fluorescence probe (sensor RB6) for Hg2+. The fluorescence probe included a fluorescein fluorophore linked to a rhodamine B hydrazide by a thiourea spacer. The color of this probe changes from yellow to magenta when reacted with Hg2+, which allows mercury detection by ratiometric fluorometry by measuring the fluorescence at a 591 nm/520 nm ratio.57 HgS is generated in the reaction due to oxadiazole cyclization after spirolactam ring opening. No application to real samples was reported. RET is an interaction between a fluorophore at the electronic excited state (energy donor) and a fluorophore at the ground state (energy acceptor), which leads to the transfer of excitation energy from the donor to the acceptor. The free fluorescence probe showed a maximum absorption wavelength at 490 nm, which exhibited a slightly yellow color dominated by the fluorescein chromophore, and no intramolecular RET phenomenon was observed. Therefore, only green fluorescence (520 nm) of fluorescein itself was observed when the probe was excited at 490 nm. The reaction of Hg2+ at the thiosemicarbazide group forces the molecule to form a 1,3,4-oxadiazole group as a new spacer and leads to the release of a fluorescent rhodamine B moiety, which triggers an intramolecular RET with a high selectivity, as shown in Scheme 12.
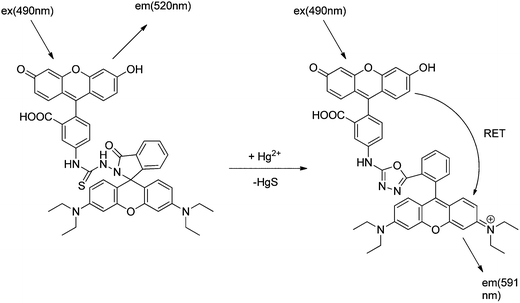 | ||
| Scheme 12 Proposed mechanism of Hg2+-induced ring opening and cyclization of the RB6 and RET-based detection mechanism of Hg2+. | ||
A rhodamine–cyclen conjugate that behaves as a highly sensitive and selective chemosensor (sensor RB7) for Hg2+ has been reported by Shiraishi et al.58 The high emission sensitivity is due to the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex leading to spirocycle opening of the sensor along with an appearance of strong orange fluorescence and a clear color change from colorless to pink. The fluorescence enhancement is 1700 and no application to real samples was reported.
2 complex leading to spirocycle opening of the sensor along with an appearance of strong orange fluorescence and a clear color change from colorless to pink. The fluorescence enhancement is 1700 and no application to real samples was reported.
New rhodamine hydrazine derivatives bearing thiol and carboxylic acid groups have been reported (sensors RB8–RB9).59 They enable the visualization of Hg2+ accumulated in the nematode C. elegans, commonly used to test and evaluate the toxicity level of heavy atoms, previously exposed to nanomolar concentrations of Hg2+.
A bis-rhodaminepiperazine conjugate has been synthesized that in the presence of Hg2+ specifically changes from colorless to pink and exhibits high fluorescence upon excitation at 520 nm (ref. 60) (sensor RB10). The presence of other relevant metal ions in the system does not affect the fluorescence output to any significant extent and the compound can be used as a chromogenic and fluorogenic sensor for Hg2+ ions in an aqueous ethanol medium, although no application to real samples was reported.
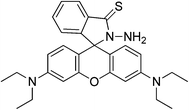
Zheng et al.61 reported a reversible Hg2+ ion sensor RB11 based on rhodamine B thiohydrazide, containing an S atom and a –NH2 group attached to the N-bearing spiro ring. The sensor RB11 is a highly selective sensor. It shows 7-fold fluorescent enhancement only in the presence of Hg2+, due to delocalization in the xanthene moiety generated after cycle rupture (Scheme 13). No application to real samples is reported to date.
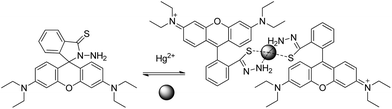 | ||
| Scheme 13 Proposed binding mode of RB11 with Hg2+. | ||
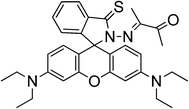
Later on, Wang et al.62 reported a fluorescent sensor based on thiooxorhodamine B (sensor RB12) to detect Hg2+ in an aqueous buffer solution. It demonstrated high selectivity for sensing Hg2+ with about 383-fold enhancement of emission intensity and micromolar sensitivity. The sensor is cell permeable and can visualize the changes in intracellular mercury ions in living cells using fluorescence microscopy, as probed by confocal fluorescence images of intracellular Hg2+ in A549 cells. A reversible mechanism based on complexation with mercuric ions is proposed (Scheme 14).
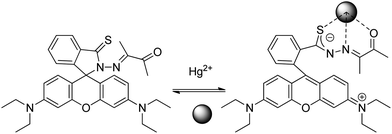 | ||
| Scheme 14 Proposed binding mode of RB12 with Hg2+. | ||
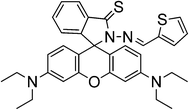
A rhodamine B based sensor (sensor RB13) was synthesized by combination of the thiospirolactam chromophore and the thiophene ring block with high affinity to Hg2+ in an aqueous solution. The introduction of the thiophene ring increases the affinity to Hg2+ ions in competitive media, changes the spatial effects within one molecule, allows real-time detection, as it quickly induces the fluorescence and color response, and improves the sensitivity of Hg2+ ions.63 Modification in the absorption spectra after the addition of Hg2+ suggests the formation of a ring-opened form. The proposed reversible mechanism, based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, is shown in Scheme 15.
1 stoichiometry, is shown in Scheme 15.
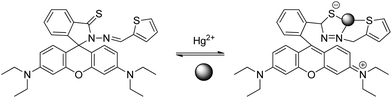 | ||
| Scheme 15 Proposed binding mode of RB13 with Hg2+. | ||
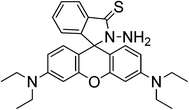
A rhodamine B thiolactone (sensor RB14) was also reported as a highly selective and sensitive sensor for Hg2+ and could be used for naked-eye detection in an aqueous solution.64,65 The change in colour can be explained to be a result of delocalization of the xanthene moiety of rhodamine due to Hg2+ binding. The chemosensor, reported by two different groups, was readily prepared and found to be stable in both alkaline and acidic solutions. The spectral response towards Hg2+ was established to be reversible by adding KI in a water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (99
CH3CN (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1% v/v) medium.65 However, in 1,4-dioxane
1% v/v) medium.65 However, in 1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (0.5
water (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99.5% v/v),64 the introduction of KI into the system can reverse the reaction only in the presence of less than 0.5 equivalents of Hg2+. The reaction scheme in this system proceeds through the route depicted in Scheme 16, in which the high thiophilicity of Hg2+ leads to the formation of two kinds of complexes of stoichiometries 2
99.5% v/v),64 the introduction of KI into the system can reverse the reaction only in the presence of less than 0.5 equivalents of Hg2+. The reaction scheme in this system proceeds through the route depicted in Scheme 16, in which the high thiophilicity of Hg2+ leads to the formation of two kinds of complexes of stoichiometries 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 R
1 R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+ and 1
Hg2+ and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 R
1 R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+. The 2
Hg2+. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex is relatively stable in solution, but the 1
1 complex is relatively stable in solution, but the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex can be further degraded to rhodamine B. No application to real samples was reported.
1 complex can be further degraded to rhodamine B. No application to real samples was reported.
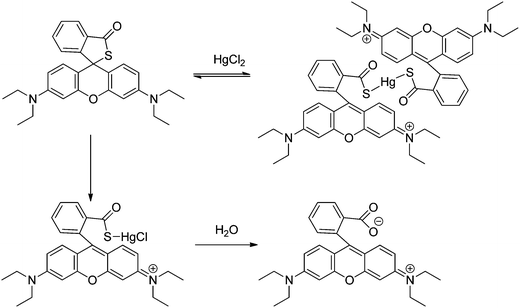 | ||
| Scheme 16 Proposed binding mode of RB14 with Hg2+. | ||
On the basis of the mechanism of Hg2+ promoted hydrolysis (Scheme 17), a new chemodosimeter has been reported (sensor RB15), and applied to Hg2+ determination in living cells with satisfactory results. The probe has a limit of detection of 0.9 ng mL−1, below the toxic levels of Hg2+ in drinking water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water reaction medium,66 and was also applied to natural water samples from three different sources: seawater from Yellow Sea (Dalian, area of China), pool water and tap water.
1 ethanol–water reaction medium,66 and was also applied to natural water samples from three different sources: seawater from Yellow Sea (Dalian, area of China), pool water and tap water.
 | ||
| Scheme 17 Proposed mechanism of Hg2+-induced ring opening and hydrolysis of RB15. | ||
4 BODIPY-based chemosensors and chemodosimeters for Hg2+
4.1 Photoinduced electron transfer (PET)
Photoinduced electron transfer (PET) sensors are an important family of chemosensors. Small molecule fluorescence turn-on sensors have been developed and studied on the basis of the PET mechanism. The general mechanism of fluorescent indicator devices based on PET combines an analyte recognition site (also called chelating group, coordination site, receptor, host or ligand) with a fluorophore, i.e. a fluorescent molecule which translates the binding between the analyte and the recognition site into a fluorescence output signal.67Changes in absorption or emission spectra are the most common ones among the possible signal modalities. In this regard, the PET process is particularly useful, as the signal, depending on the special circumstances, is either an “on–off” or “off–on” type,68,69 resulting in a well-defined response. An interesting fact is that PET produces very sharp changes in the signal intensity, while keeping the emission wavelength unchanged.
For PET sensors the receptor and fluorophore are usually separated by a (short) spacer electronically connecting the π-electron systems of receptor and fluorophore. In such systems, the receptors usually contain a relatively high-energy nonbonding electron pair. In the unbound state, after excitation, an electron of the highest occupied molecular orbital (HOMO) is promoted to the lowest unoccupied molecular orbital (LUMO), which enables a fast intramolecular electron transfer from the HOMO of the receptor (donor) to the LUMO of the excited fluorophore. Such a process provides a mechanism for nonradiative deactivation of the excited state, causing a quenching effect of the fluorescence of the system. When the receptor is bound, this electron pair is coordinated to Lewis acid cations in solution, the HOMO of the receptor is lowered becoming lower than that of the fluorophore, the receptor redox potential is perturbed and slows down the PET process, reviving fluorescence emission; this logic indication can also be reversed.70
Fig. 1 shows a three module format in which a reductive PET is produced by permitting electron transfer from the receptor to the fluorophore, if the process is thermodynamically and kinetically feasible.67 The electron transfer rate in many favorable cases is much faster than the luminescence rate when PET is thermodynamically allowed. Luminescence and electron transfer are the two main competitors, which deactivate the photoexcited state of these designed systems. Binding of the target species (typically a metal ion) to the receptor can drastically alter PET thermodynamics to an endergonic situation. At the simplest level, this situation is caused by electrostatic interactions between the receptor–target pair. Luminescence is now the winner of the competition and thus can be switched between “on” and “off” states by introduction and removal, respectively, of the target species, which provides us with the responsive mechanism we needed (Fig. 1). Mechanistically, the fluorophore–receptor pair (Fig. 1A) is selected to allow rapid PET between them on the basis of simple electrochemical criteria. The rapidity is usually assured by sufficiently favorable PET thermodynamics and by using sufficiently short spacers. However, the designer must also allow for the reversal of PET thermodynamics in the guest-bound situation (Fig. 1B). Guest-induced conformational changes in the receptor can be valuable adjunct to purely electrostatic effects for the facilitation of sensing. The clearest result is a fluorescence switching “off–on” effect caused by the guest.67
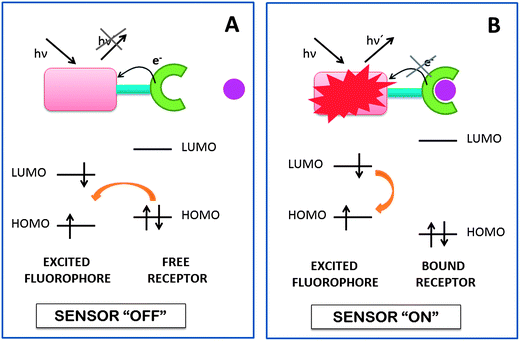 | ||
| Fig. 1 Reductive PET for fluorescent indicators. (A) The three module format allows electron transfer from the chelator to the fluorophore, switching off the sensor. (B) When the receptor is bound, the receptor redox potential is perturbed and slows down the PET process, switching on the sensor. | ||
4.2 BODIPY mechanism for Hg2+ chemosensing and derivatives
Since their discovery in 1968 by Treibs et al.,71 BODIPY dyes (4,4-difluoro-4-bora-3a, 4a-diaza-s-indacene, difluoroborondipyrromethene) have become very popular in the fluorescent chemosensor field due to their notable properties, starting from the relatively moderate redox potential of the BODIPY core (see Fig. 2), which is a requisite in the construction of fluorescent probes based on electron transfer processes.70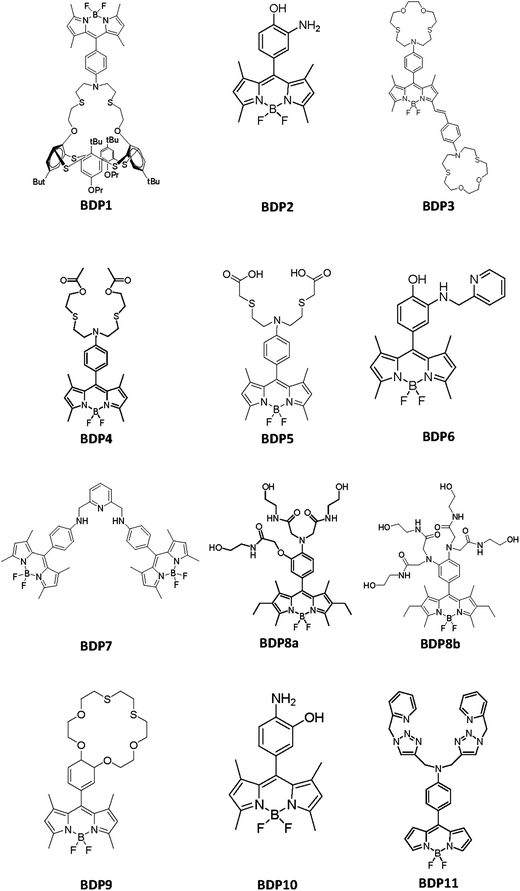 | ||
| Fig. 2 Chemical structures of the BODIPY derivatives included in Table 3. | ||
The excellent luminescent properties of the BODIPY dyes included their photochemical stability, the relatively high molar absorption coefficients and fluorescence quantum yields, insignificant triplet-state formation, excitation and emission wavelengths in the visible spectral region with high intensity narrow emission peaks, good solubility and resistance toward self-aggregation in solution and fluorescence lifetimes in the nanosecond range.72–74
Chemosensors based on the BODIPY system follow the mechanism showed on Scheme 18. The fluorophore is difluoroborondipyrromethene. It is connected to the aromatic ring that acts as a spacer from the receptor moiety. The aromatic ring includes a second substituent in the para position that is a strong electron-donor (amino, hydroxyl or ether group, usually) and in the absence of the analyte inhibits fluorescent emission. The receptor system, indicated by dashed lines in Scheme 18, can have other groups with lone electron pairs, and/or suitable coordination geometry that increase selectivity to the target of interest. When complexation with the analyte is produced, electron transmission to the fluorophore is disconnected, and then it shows its fluorescent properties.
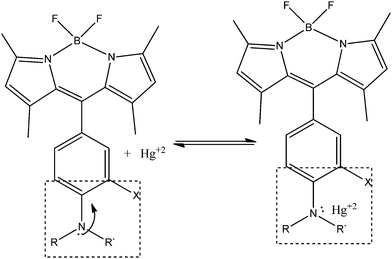 | ||
| Scheme 18 Mechanism of BODIPY PET sensors for Hg2+ determination. | ||
Moreover, their versatile chemistry allows the core derivatization in a variety of ways75–77 leading to the generation of a multitude of chemosensors selectively to a wide range of metal cations of biological and environmental interest.78 The attachment of adequate residues in several positions of the BODIPY core allows the building of sensors with specific spectroscopic and photophysical properties.79
To the best of our knowledge, Table 3 contains the properties of the BODIPY-based chemosensors able to detect Hg2+ following a PET mechanism, although in some cases, an intramolecular charge transfer (ICT) mechanism also exists, some of them proving to be very effective in concrete analytical applications. To see their chemical structures, please refer to Fig. 2.
| Sensor compound | ∅ | [R] (μM) | Reaction medium | λ ex (nm) | λ em (nm) | LOD (ng mL−1) | Interferences | R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+ stoichiometry Hg2+ stoichiometry |
Mechanism | Analytical applications | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDP1 | 0.062 | 10 | CH3CN | 490 | 529 | — | Cu2+, Fe3+, Ag+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET | — | 79 |
| BDP2 | 0.01 | 5 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer 1 HEPES buffer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 (v/v), pH = 7.2 99 (v/v), pH = 7.2 |
483 | 510 | — | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET | — | 74 |
| BDP3 | 0.33 | 2 | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer 30 HEPES buffer 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (v/v), pH = 7.2 70 (v/v), pH = 7.2 |
540 | 578 | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET and ICT | — | 80 |
| BDP4 | 0.58 | 1 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CH2OH, 7 CH3CH2OH, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v), pH = 7.0 3 (v/v), pH = 7.0 |
490 | 512 | — | Ag+, Cl−, Br−, CO32−, SCN−, CH3CO2− | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET | — | 81 |
| BDP5 | 0.29 | 2 | HEPES buffer, pH = 7.2 | 480 | 507 | 15 | Ag+, Pb2+, Cu2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET | Living cells | 82 |
| BDP6 | 0.054 | 0.5 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer 1 HEPES buffer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 (v/v), pH = 7.2 99 (v/v), pH = 7.2 |
470 | 509 | 2 | Ag+, Pb2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
PET | Living cells | 84 |
| BDP7 | 0.13 | 5 | CH3OH | 483 | 513 | — | Al3+, Fe2+ | — | PET | — | 85 |
| BDP8a, BDP8b | 0.19, 0.61 | 2 | Phosphate (0.1 M) solution, pH = 7.5 | 527, 527 | 538, 541 | 2 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
PET, PET | — | 86 |
| BDP9 | — | 0.5 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN, 60 CH3CN, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v) 40 (v/v) |
498 | 507 | — | Ag+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET | — | 88 |
| BDP10 | — | 10 | CH3CH2OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer (20 mM HEPES, 100 mM NaNO3, 1 HEPES buffer (20 mM HEPES, 100 mM NaNO3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(v/v), pH = 7.0) 1(v/v), pH = 7.0) |
495 | 513 | ≤2 | Ag+, Cu2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET | Natural waters | 89 |
| BDP11 | 0.035 | 30 | CH3OH | 492 | 520 | 5.6 × 102 | Co2+, Fe3+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PET | Living cells | 90 |
A chemosensor containing a thiacalix[4](N-phenylazacrown-5)ether ligand linked by a spacer to a BODIPY core was described by Bitter and coworkers (BDP1, in Table 3).79 Although the binding of Hg2+ ions results in an emission enhancement, the pronounced off–on response due to the PET process is generated in the presence of Cu2+, and Fe3+ to a lower extent. In the presence of Ag+, the latter response is diminished. No application to real samples was reported.
A BODIPY derivative with an o-aminophenol chelator at the meso-position which acts as a specific fluorescence turn-on sensor for Hg2+ and a selective colorimetric chemosensor for Cu2+ has been reported (BDP2, in Table 3).74 After the addition of Hg2+, the low quantum yield (∅ < 0.002) is increased to 0.01, with no interference of a wide variety of other ions. No application to real samples was reported to date.
M. Yuan et al. have reported the synthesis and sensing characteristics of a new class of colorimetric and fluorimetric dual-channel assay for the specific detection of Hg2+ in the presence of other cations (BDP3, in Table 3) that comprises both PET and ICT processes in a single molecule. In the absence of Hg2+ the BDP3 molecule is non fluorescent due to an efficient PET process. The latter is modified after the addition of Hg2+, leading to an increment of the fluorescence intensity of ca. 7-folds.80 No application to real samples was reported.
Du and coworkers have synthesized and characterized a new highly selective fluorescent sensor with an open-chain azadioxadithia (NO2S2) chelator for Hg2+ determination (BDP4, in Table 3). Without Hg2+ it has a low ∅, due to an efficient reductive PET from the NO2S2 chelator to BODIPY. A large fluorescent enhancement (160-fold) is produced after the addition of Hg2+ caused by the inhibition of the PET mechanism. Because of the great coordinating ability of Cl−, Br−, CO32−, SCN− and CH3CO2− to Hg2+, the bright fluorescence of the Hg2+–sensor complex is affected by these anions.81 No application to real samples was reported.
A fluorescent chemosensor which exhibits selective fluorescence towards Hg2+ was presented by Fan and collaborators (BDP5, in Table 3). In contrast to sensor BDP4, it is unaffected by the presence of anions existing in its environment and organisms. By examining the application of BDP5 to PC12 cultured cells, it was observed that BDP5 could not permeate through the cell membrane. According to that, a BDP5-ester derivative non-toxic to cell cultures was used to get into cells and be transformed in BDP5 by esterase in the cytosol. The latter modification allows BDP5 to image intracellular Hg2+ in living cells, providing a useful way to study its toxicity and bioactivity.82
Lu et al. reported a fluorescent turn-on chemosensor able to selectively determine Hg2+ not only in aqueous media, but also within living cells (BDP6, in Table 3). In aqueous media, this sensor presents high sensitivity towards Hg2+, reaching detection limits of ≤2 ng mL−1, which is the U.S. Environmental Protection Agency's limit for drinking water.83,84 Regarding living cells, experiments were conducted in HeLa cells by incubating them with BDP6 after addition of Hg2+. Fluorescence determination with laser scanning confocal microscopy showed a significant fluorescence emission from the perinuclear region of the cytosol, indicating that BDP6 was cell-permeable, could respond to variations in intracellular Hg2+ and would be used to monitor Hg2+ and study its effects within living cells.84
Lu and collaborators have developed a BODIPY-based fluorescence probe highly selective to Hg2+ in methanol (BDP7, in Table 3). The theoretical and experimental characterizations of the turn-on response in the presence of Hg2+ reveal that the enhancement is due to a prohibited reductive and oxidative PET processes, which allow the fluorophore to emit a strong fluorescence.85 No application to real samples was reported.
Three fluorescent sensor molecules with polyamide receptors were designed and synthesized by Wang and collaborators, two of them having high sensitivity towards Hg2+. The very weak basal fluorescence of BDP8a and BDP8b is largely enhanced after the addition of Hg2+, because the reductive PET quenching process is impeded due to the positively cooperative metal–sensor complexation. The 2 ng mL−1 of Hg2+ detected using the molecule with the tetraamide receptor, in addition to the sensor's high water solubility, allow the development of probes useful to monitor low levels of Hg2+ in drinking waters.86 The tetramide substituents act as electron donors, and upon excitation of the fluorophore, an electron transfer occurs; then the fluorescence is diminished when the fluorescent group in its excited-state is reduced. In this situation, the fluorescent group is acting as an acceptor and displays very weak fluorescence, resulting from the efficient PET quenching process from the electron-donating receptor moiety to the excited BODIPY fluorophore. In the presence of Hg2+ binding to the recognition tetramide moiety where the donor atom is present, the PET quenching pathway is efficiently blocked and an OFF–ON enhancement of the fluorescence is produced. The clear emission turn-on response is observed when concentrations as low as ng mL−1 level of Hg2+ are present and the reported molecular probe is able to act as an efficient and selective chemosensor unit for environmental Hg2+ monitoring. The high selectivity of the method towards other metal ions was corroborated by an interference study and the chemosensor unit is of potential application as a fluorescence sensor intended for in situ analysis by appropriate immobilization.87
A BODIPY appended thiacrown molecule able to detect Hg2+ and Ag+ in a H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN medium has been reported (BDP9, in Table 3). The involved PET mechanism is blocked in the presence of Hg2+, which interacts with the sulfur atoms of the thiacrown part.88
CH3CN medium has been reported (BDP9, in Table 3). The involved PET mechanism is blocked in the presence of Hg2+, which interacts with the sulfur atoms of the thiacrown part.88
Fan et al. have developed a BODIPY derivative turn-on sensor able to sensitively and selectively detect Hg2+ even in the presence of cysteine or in sulfur-rich environments, which are known to form stable complexes with Hg2+ (BDP10, in Table 3). The aminophenol ligand added to the BODIPY is responsible for the efficient PET process (∅ = 0.3%), which reverts in the presence of Hg2+ with a 20-fold fluorescence intensity increase. This probe was successfully applied to the quantitation of Hg2+ at low levels in different kinds of natural water samples.89
A new chemosensor capable of selectively detecting Hg2+ over competing metal ions was designed by Vedamalai and Wu (BDP11, in Table 3). After the chelation of Hg2+ through the triazole units, the PET mechanism is blocked, leading to a great fluorescence enhancement. Experiments in HeLa cells were carried out in order to sense Hg2+ ions in living cells. Images gathered with a fluorescence microscope showed that the fluorescent signal was located in the intracellular area, which indicates a subcellular distribution of Hg2+ and a good permeability of BDP11.90
4.3 BODIPY based chemodosimeters
Khan and Ravikanth reported a 3-(pyridine-4-thione)BODIPY (BDP12 in Scheme 19) which can be used as a selective but not sensible, i.e. limit of detection of 3 × 106 ng L−1, chemodosimeter for detecting Hg2+. The latter was designed taking into account the great affinity between Hg2+ and sulfur, which allows a desulfurization reaction that is traduced in significant changes in the fluorescent properties of the chemodosimeter. In fact, the very low emission signal of BDP12 is increased after the addition of Hg2+ ions due to its desulfurization.91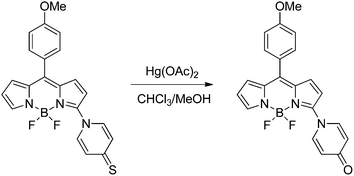 | ||
| Scheme 19 Induced desulfuration reaction of BDP12 in the presence of Hg2+. | ||
A dual channel chemodosimeter for Hg2+ and Ag+ detection was developed by Zhang and collaborators based on the concept of aldehyde group protection/deprotection. In the absence of Hg2+ the emission fluorescence of BODIPY-1,3-dithiane (BDP13 in Scheme 20) is very weak due to the PET caused by the sulfur on the thioacetal moiety. After the addition of Hg2+ a deprotection reaction of thioacetal occurs, which allows the enhancement of the basal fluorescence of BDP13 with a blue shift in the emission maximum and limit of detection of 2.8 × 107 ng L−1. On the other hand, Ag+ did not desulfurize the thioacetal of BDP13. Instead of that, it coordinates with the sulfur atoms of the thioacetal moiety inhibiting the PET quenching of fluorescence and leading to the restoration of the emission without wavelength shift. Thus, this constitutes a BODIPY-based probe with dual channel fluorescence for two different metal ions.92
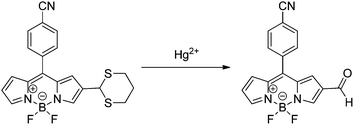 | ||
| Scheme 20 Dethioacetalization reaction of BDP13 in the presence of Hg2+. | ||
5 Immobilization of molecular probes in solid supports
A new trend in chemical sensing is based on the use of new materials for molecular probe immobilization. Polymers, crystals, glasses, particles, gold nanoparticles and nanostructures have made a profound impact on modern sensory systems, and the design and synthesis of novel materials with controlled sensing properties is a significant challenge within nanoscience and nanotechnology. However, there are only a few recent examples that concern Hg2+ detection.93In a recent paper, double hydrophilic block co-polymers bearing rhodamine B-based Hg2+ reactive moieties were reported, which can serve as multifunctional sensors to pH, temperature and Hg2+ ions.94 The block was composed of poly(ethylene oxide)-b-poly(N-isopropylacrylamide-co-RhBHA) (PEO-b-P(NIPAM-co-RhBHA)). Non-fluorescent RhBHA moieties were subjected to selective ring-opening reaction upon addition of Hg2+ ions and pH, producing highly fluorescent acyclic species. Upon heating above the lower critical solution temperature (36 °C) of the PNIPAM block, they self-assemble into micelles possessing P(NIPAM-co-RhBHA) cores and well-solvated PEO coronas. It was found that the detection sensitivity to Hg2+ ions and pH could be dramatically improved at elevated temperatures due to fluorescence enhancement of RhBHA residues in the acyclic form, which were embedded within hydrophobic cores of the thermo-induced micellar aggregates.
In a second report,95 novel fluorescent silica nanoparticles, formed by quantum dot SiO2-RH 6G nanoparticles, with high selectivity towards Hg2+, were synthesized for the detection of Hg2+. The mercury ions promote the ring opening of spirolactam in the rhodamine moiety grafted onto the silica nanoparticles, resulting in a change in the fluorescence intensity. In this process, a Hg2+ ion takes part in the complexation with an oxygen atom of the rhodamine carbonyl group, resulting in the enhancement of the fluorescence intensity at 545 nm. When KI is added, the opened spirolactam is closed again, which can be attributed to the stronger binding ability of I− than Hg2+. A LOD of 0.52 ng mL−1 was reported with a linear range from 8.0 to 160 ng mL−1, and the proposed approach was successfully applied to the determination of Hg2+ in tap and lake water samples with satisfactory results.
Finally, a highly sensitive and selective probe, sensor RG4 (ref. 44 and 45) (see Table 1), was proposed for the fluorimetric determination of mercury ion traces in aqueous solutions. The probe was based on the mercury-promoted ring opening of the spirolactam moiety of a rhodamine 6G spirocyclicphenylthiosemicarbazide derivative, retained in nylon membranes (Scheme 21). It was demonstrated that the chemodosimeter preserves its sensor ability, displaying intense fluorescence in the presence of Hg2+ after being immobilized on the nylon surface and reacting with the mercury ion solution via a simple syringe procedure. The advantages of this proposal are: (1) the use of an easily affordable solid support which is able to immobilize the molecular probe without involving a covalent bond, (2) the consumption of a very small volume of organic reagent, dramatically reducing the environmental impact, and (3) the development of a solid phase system potentially useful as a main component for designing chemical sensors capable of providing continuous real-time information. Thus, a simple and sensitive fluorescence method for the determination of mercury ions was established. The limit of detection was 0.4 ng mL−1 (lower than the toxic levels in drinking water for human consumption, established by several regulatory agencies), and the sampling rate was about 15 samples per hour. The study of the potential interference from common cations demonstrated a remarkable selectivity for the investigated metal ions. The viability of determining Hg2+ residues in real water samples was successfully evaluated through the recovery study of several environmental water samples from different locations.
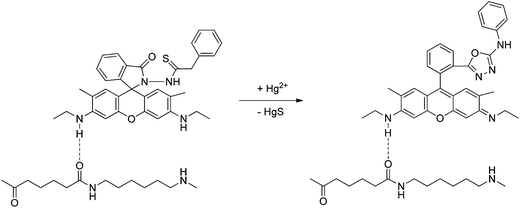 | ||
| Scheme 21 Proposed mechanism of Hg2+-induced ring opening and cyclization of RG4 retained in nylon membranes. | ||
The same rhodamine derivative, sensor RG4, was also used to develop a novel Hg2+ fluorescent polymeric sensor phase to measure Hg2+ in solution, with high sensitivity and selectivity, in a quick and easy way.46 The co-polymers were synthesized by the reverse ATRP technique, based on the copolymerization of methylmethacrylate (MMA) and HEMA, following a previously reported procedure.96 To prepare the optimal sensing film, a mixture of co-polymer, plasticizer, and RG4 dye was prepared in acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (80
methanol (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v). The Hg2+-sensitive sensing film was made by drop coating on poly(ethylene terephthalate) and was successfully applied for Hg2+ monitoring, showing high selectivity and relatively low response times (less than 30 minutes).
20 v/v). The Hg2+-sensitive sensing film was made by drop coating on poly(ethylene terephthalate) and was successfully applied for Hg2+ monitoring, showing high selectivity and relatively low response times (less than 30 minutes).
6 Conclusions
The development of efficient, selective and sensitive methods for monitoring Hg2+, in environmental and biological systems, has been stimulated due to the alarm for the worldwide spread of mercury pollution and its damage to human health and the environment. A number of highly selective and sensitive fluorescent chemodosimeters and chemosensors, using rhodamine and BODIPY derivatives, have been exploited for in vivo monitoring of Hg2+ and for the detection of Hg2+ in environmental samples. Several limitations such as low water solubility, poor selectivity toward other metal ions, and weak sensitivity in physiological conditions prevent the sensor molecules being applicable in biological systems in some cases. Only a few of the above reported works discussed analytical figures of merit and few fluorescent probes work in pure aqueous solutions, and a number of the reported probes have not been applied in real samples. The summarized reports are conclusive of being a very active area of research and that there is a need for working on the design and application of fluorescent probes that may work in pure aqueous solutions at physiological pH. Development of such sensors will help to solve many problems of trace-level toxic Hg2+ determination, in different biological and environmental samples, in a simple and rapid way. The combination of Hg2+ responsive polymers and other immobilization structures and nanostructures, with well developed small molecules based on selective sensing moieties, are expected to exhibit preferred advantages, including enhanced detection sensitivity, biocompatibility, facile integration into devices and the ability of further functionalization for targeted delivery and imaging. The preparation of nanostructured and/or polymeric sensing films, with reduced response times, in combination with optical fibers, is a fundamental step for the development of practical sensor optrodes, applicable for real time and in situ monitoring.Acknowledgements
The authors gratefully acknowledge Universidad Nacional de Litoral, CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica), Ministerio de Economía y Competitividad of Spain (Project CTQ2011-25388), and the Gobierno de Extremadura (Consolidation Project GR10033 of Research Group FQM-003), both co-financed by European FEDER funds, for financially supporting this work.References
- L. Magos, Physiol. Toxicol. Mercury, 1997, 34, 321–370 Search PubMed.
- M. F. Wolfe, S. Schwarzbach and R. A. Sulaiman, Environ. Toxicol. Chem., 1998, 17, 146–160 CrossRef CAS.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS.
- P. Grandjean, P. Weihe, R. F. White and F. Debes, Environ. Res., 1998, 77, 165–172 CrossRef CAS.
- S. H. Choi, K. Pang, K. Kim and D. G. Churchill, Inorg. Chem., 2007, 46, 10564–10577 CrossRef CAS.
- A. Renzoni, F. Zinoand and E. Franchi, Environ. Res., 1998, 77, 68–72 CrossRef CAS.
- Mercury Update: Impact on Fish Advisories. EPA Fact Sheet EPA-823-F-01–011, EPA, Office of Water, Washington, DC, 2001 Search PubMed.
- R. Von Burg and M. R. Greenwood, in Metals and their Compounds in the Environment, ed. E. Merian, VCH, Weinheim, 1991, pp. 1045–1088 Search PubMed.
- S. Atilgan, I. Kutuk and T. Ozdemir, Tetrahedron Lett., 2010, 51, 892–894 CrossRef CAS.
- D. W. Boening, Chemosphere, 2000, 40, 1335–1351 CrossRef CAS.
- H. H. Harris, I. Pickering and G. N. George, Brevia: the Chemical Form of Mercury in Fish Science, Wash. D.C., 2003, vol. 301(5637), p. 1203 Search PubMed.
- S. D. Richardson and T. A. Temes, Anal. Chem., 2005, 77, 3807–3838 CrossRef CAS.
- X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025–8029 CrossRef CAS.
- T. Takeuchi, N. Morikawa, H. Matsumoto and Y. Shiraishi, Acta Neuropathol., 1962, 2, 40–57 CrossRef.
- M. Harada, Crit. Rev. Toxicol., 1995, 25, 1–24 CrossRef CAS.
- R. Von Burg, J. Appl. Toxicol., 1995, 15, 483–493 CrossRef CAS.
- T. W. Clarkson, L. Magos and G. J. Myers, N. Engl. J. Med., 2003, 349, 1731–1737 CrossRef CAS.
- B. Welz and M. Sperling, Atomic Absorption Spectrometry, Weinheim, Germany, Wiley, 3rd edn, 1999 Search PubMed.
- Y. Gao, Z. Shi, Z. Long, P. Wu, C. Zheng and X. Hou, Microchem. J., 2012, 103, 1–14 Search PubMed.
- A. Q. Shah, T. G. Kazi, J. A. Baig, H. I. Afridi and M. B. Arain, Food Chem., 2012, 134, 2345–2349 CrossRef CAS.
- J. A. Moreton and H. T. Delves, J. Anal. At. Spectrom., 1998, 13, 659–665 RSC.
- J. Djedjibegovic, T. Larssen, A. Skrbo, A. Marjanovic and M. Sober, Food Chem., 2012, 131, 469–476 Search PubMed.
- E. Kenduzler, M. Ates, Z. Arslan, M. McHenry and P. B. Tchounwou, Talanta, 2012, 93, 404–410 CrossRef CAS.
- Y. Zhao, J. Zheng, L. Fang, Q. Lin, Y. Wu, Z. Xue and F. Fu, Talanta, 2012, 89, 280–285 CrossRef CAS.
- Y. Yin, M. Chen, J. Peng, J. Liu and G. Jiang, Talanta, 2010, 81, 1788–1792 CrossRef CAS.
- J. L. Rodrigues, S. S. de Souza, V. C. de Oliveira Souza and F. Barbosa Jr, Talanta, 2010, 80, 1158–1163 CrossRef CAS.
- X. Jia, Y. Han, X. Liu, T. Duan and H. Chen, Spectrochim. Acta, Part B, 2011, 66, 88–92 CrossRef.
- A. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- M. Dutta and D. Das, TrAC, Trends Anal. Chem., 2012, 32, 113–132 CrossRef CAS.
- V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386–7387 CrossRef CAS.
- D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280–6301 CrossRef CAS.
- R. Von Burg, J. Appl. Toxicol., 1995, 15, 483–493 CrossRef CAS.
- J. S. Wu, I. C. Hwang, K. S. Kim and J. S. Kim, Org. Lett., 2007, 9, 907–910 CrossRef CAS.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- J. F. Zhang and J. S. Kim, Anal. Sci., 2009, 25, 1271–1281 CrossRef CAS.
- D. Wu, W. Huang, C. Duan, Z. Lin and Q. Meng, Inorg. Chem., 2007, 46, 1538–1540 CrossRef CAS.
- W. Huang, P. Zhou, W. Yan, C. He, L. Xiong, F. Li and C. Duan, J. Environ. Monit., 2009, 11, 330–335 RSC.
- J. S. Wu, I. C. Hwang, K. S. Kim and J. S. Kim, Org. Lett., 2007, 9, 907–910 CrossRef CAS.
- Y. K. Yang, K. J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760–16761 CrossRef CAS.
- S. K. Ko, Y. K. Yang, J. Tae and I. Shin, J. Am. Chem. Soc., 2006, 128, 14150–14155 CrossRef CAS.
- Y. K. Yang, S. K. Ko, I. Shin and J. Tae, Nat. Protoc., 2007, 2, 1740–1745 Search PubMed.
- Y. K. Yang, S. K. Ko, I. Shin and J. Tae, Org. Biomol. Chem., 2009, 7, 4590–4593 RSC.
- D. Bohoyo Gil, M. I. Rodríguez-Cáceres, M. C. Hurtado-Sánchez and A. Muñoz de la Peña, Appl. Spectrosc., 2010, 64, 520–527 CrossRef.
- A. Muñoz de la Peña, M. I. Rodríguez Cáceres, M. C. Hurtado-sánchez and D. Bohoyo Gil, Luminescence, 2010, 25, 229–230 Search PubMed.
- V. A. Lozano, G. M. Escandar, M. C. Mahedero and A. Muñoz de la Peña, Anal. Methods, 2012, 4, 2002–2008 RSC.
- F. J. Orriach-Fernández, A. L. Medina-Castillo, J. F. Fernández-Sánchez, A. Muñoz de la Peña and A. Fernández-Gutierrez, Proceedings of the Europtrode XI Conference on Optical Chemical Sensors and Biosensors, 2012, Barcelona, Spain, Communication P-36, p. 102 Search PubMed.
- P. Xi, L. Huang, H. Liu, P. Jia, F. Chen, M. Xu and Z. Zeng, JBIC, J. Biol. Inorg. Chem., 2009, 14, 815–819 CrossRef CAS.
- D. Wu, W. Huang, Z. Lin, C. Duan, C. He, S. Wu and D. Wang, Inorg. Chem., 2008, 47, 7190–7201 CrossRef CAS.
- X. Chen, S. W. Nam, M. J. Jou, Y. Kim, S. J. Kim, S. Park and J. Yoon, Org. Lett., 2008, 10, 5235–5328 CrossRef CAS.
- A. Muñoz de la Peña, M. I. Rodríguez Cáceres, D. Bohoyo Gil, M. C. Mahedero, M. C. Hurtado-Sánchez and R. Babiano, Am. J. Anal. Chem., 2011, 2, 605–611 Search PubMed.
- W. Lin, X. Cao, Y. Ding, L. Yuan and L. Long, Chem. Commun., 2010, 46, 3529–3531 RSC.
- D. T. Quang, J. S. Wu, N. D. Luyen, T. Duong, N. D. Dan, N. C. Bao and P. T. Quy, Spectrochim. Acta, Part A, 2011, 78, 753–756 CrossRef.
- M. H. Lee, J. S. Wu, J. W. Lee, J. H. Jung and J. S. Kim, Org. Lett., 2007, 9, 2501–2504 CrossRef CAS.
- J. H. Soh, K. M. K. Swamy, S. K. Kim, S. Kim, S. H. Lee and J. Yoon, Tetrahedron Lett., 2007, 48, 5966–5969 CrossRef CAS.
- H. Yang, Z. Zhou, K. Huang, M. Yu, F. Li, T. Yi and C. Huang, Org. Lett., 2007, 9, 4729–4732 CrossRef CAS.
- J. Huang, Y. Xu and X. Quian, J. Org. Chem., 2009, 74, 2167–2170 CrossRef CAS.
- G. Q. Shang, X. Gao, M. X. Chen, H. Zheng and J. G. Xu, J. Fluoresc., 2008, 18, 1187–1192 CrossRef CAS.
- Y. Shiraishi, S. Sumiya, Y. Kohno and T. Hirai, J. Org. Chem., 2008, 73, 8571–8574 CrossRef CAS.
- H. N. Kim, S. W. Nam, K. M. K. Swamy, Y. Jin, X. Chen, Y. Kim, S. J. Kim, S. Park and J. Yoon, Analyst, 2011, 136, 1339–1343 RSC.
- S. B. Maity and P. K. Bharadwaj, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2011, 50, 1298–1302 Search PubMed.
- H. Zheng, Z. H. Qian, L. Xu, F. F. Yuan, L. D. Lan and J. G. Xu, Org. Lett., 2006, 8, 859–861 CrossRef CAS.
- H. H. Wang, L. Xue, C. L. Yu, Y. Y. Qian and H. Jiang, Dyes Pigm., 2011, 91, 350–355 CrossRef CAS.
- Y. Zhou, X. Y. You, Y. Fang, J. Y. Li, K. Liu and C. Yao, Org. Biomol. Chem., 2010, 8, 4819–4822 RSC.
- W. Shi and H. Ma, Chem. Commun., 2008, 1856–1858 RSC.
- X. Q. Zhan, Z. H. Quian, H. Zheng, B. Y. Su, Z. Lamb and J. G. Xu, Chem. Commun., 2008, 1859–1861 RSC.
- J. Du, J. Fan, X. Peng, P. Sun, J. Wang, H. Li and S. Su, Org. Lett., 2010, 12, 476–479 CrossRef CAS.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- A. P. de Silva and N. D. McClenaghan, J. Am. Chem. Soc., 2000, 122, 3965–3966 CrossRef CAS.
- N. Kaur, N. Singh, D. Cairns and J. F. Callan, Org. Lett., 2009, 11, 2229–2232 CrossRef CAS.
- H. Lu, S. Zhang, H. Liu, Y. Wang, Z. Shen, C. Liu and X. You, J. Phys. Chem. A, 2009, 113, 14081–14086 CrossRef CAS.
- A. Treibs, F. H. Kreuzer and J. Liebigs, Justus Liebigs Ann. Chem., 1968, 718, 208–223 CrossRef CAS.
- R. P. Haugland, Handbook of Fluorescent Probes and Research Chemicals, Molecular Probes, Eugene, OR, USA, 9th edn, 2002 Search PubMed.
- K. Rurack, M. Kollmannsberger, U. Resch-Genger and J. Daub, J. Am. Chem. Soc., 2000, 122, 968–969 CrossRef CAS.
- H. Lu, Z. Xue, J. Mack, Z. Shen, X. You and N. Kobayashi, Chem. Commun., 2010, 46, 3565–3567 RSC.
- O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029–8036 CrossRef CAS.
- T. Rohand, M. Baruah, W. W. Qin, N. Boens and W. Dehaen, Chem. Commun., 2006, 266–268 RSC.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- V. Csokai, M. Kádar, D. L. H. Mai, O. Varga, K. Tóth, M. Kubinyi, A. Grün and I. Bitter, Tetrahedron, 2008, 64, 1058–1063 CrossRef CAS.
- M. Yuan, Y. Li, J. Li, C. Li, X. Liu, J. Lv, J. Xu, H. Liu, S. Wang and D. Zhu, Org. Lett., 2007, 9, 2313–2316 CrossRef CAS.
- J. Du, J. Fan, X. Peng, H. Li, J. Wang and S. Sun, J. Fluoresc., 2008, 18, 919–924 CrossRef CAS.
- J. Fan, K. Guo, X. Peng, J. Du, J. Wang, S. Sun and H. Li, Sens. Actuators, B, 2009, 142, 191–196 CrossRef.
- National Primary Drinking Water Regulations, EPA 816-F-09-0004, May, 2009.
- H. Lu, L. Xiong, H. Liu, M. Yu, Z. Shen, F. Li and X. You, Org. Biomol. Chem., 2009, 7, 2554–2558 RSC.
- H. Lu, S. Zhang, H. Z. Liu, Y. W. Wang, Z. Shen, C. G. Liu and X. Z. You, J. Phys. Chem. A, 2009, 113, 14081–14086 CrossRef CAS.
- J. Wang and X. Qian, Org. Lett., 2006, 8, 3721–3724 CrossRef CAS.
- A. Muñoz de la Peña, A. Machuca, R. Babiano Caballero, J. M. Culzoni and H. C. Goicoechea, Proceedings of the Europtrode XI Conference on Optical Chemical Sensors and Biosensors, 2012, Barcelona, Spain, Communication P-23, p. 89 Search PubMed.
- H. J. Kim, S. H. Kim, J. H. Kim, E. Lee, K. Kim and J. S. Kim, Bull. Korean Chem. Soc., 2008, 29, 1831–1834 CrossRef CAS.
- J. Fan, X. Peng, S. Wang, X. Liu, H. Li and S. Sun, J. Fluoresc., 2012, 22, 945–951 Search PubMed.
- M. Vedamalai and S. Wu, Eur. J. Org. Chem., 2012, 1158–1163 CrossRef CAS.
- T. K. Khan and M. Ravikanth, Dyes Pigm., 2012, 95, 89–95 Search PubMed.
- X. Zhang, Y. Xu, P. Guo and X. Qian, New J. Chem., 2012, 36, 1621–1625 RSC.
- Y. M. Jian, L. Y. Jun, L. H. Biao and L. Y. Liang, Sci. China, Ser. B: Chem., 2009, 52, 715–730 CrossRef CAS.
- J. Hu, C. Li and S. Liu, Langmuir, 2010, 26, 724–729 CrossRef CAS.
- H. Liu, P. Yu, D. Du, C. He, B. Qin, X. Chen and G. Chen, Talanta, 2010, 81, 433–437 CrossRef.
- A. L. Medina-Castillo, J. F. Fernández-Sánchez, A. Segura-Carretero and A. Fernández-Gutiérrez, J. Mater. Chem., 2011, 21, 6742–6750 RSC.
| This journal is © The Royal Society of Chemistry 2013 |