Determination of bisphenol A, bisphenol F and their diglycidyl ethers in environmental water by solid phase extraction using magnetic multiwalled carbon nanotubes followed by GC-MS/MS
Yanna
Jiao
ab,
Li
Ding
a,
Shanliang
Fu
a,
Shaohua
Zhu
a,
Hui
Li
b and
Libing
Wang
*a
aHunan Entry–Exit Inspection and Quarantine Bureau of the People's Republic of China, Changsha, 410004, P R China. E-mail: wanglb0419@126.com; Fax: +86-73185627820; Tel: +86-73185627812
bCollege of Chemical Engineering, Sichuan University, Chengdu, 610065, P R China
First published on 5th December 2011
Abstract
The magnetic multiwalled carbon nanotube (MWCNT-MNP) was successfully synthesized. MWCNT-MNP and gas chromatography-tandem mass spectrometry (GC-MS/MS) were used for rapid, simple and reliable determination of bisphenol A (BPA), bisphenol F (BPF), bisphenol F diglycidyl ether (BFDGE) and bisphenol A diglycidyl ether (BADGE) at trace levels in water. The major factors affecting the recovery efficiency, such as amount of MWCNT-MNP, pH of water sample and sample volume, were carefully investigated. The recovery of these compounds was in the range of 88.5–115.1% with relative standard deviation less than 10%. Good linearities (r2 > 0.995) were obtained. The limits of detection for BPA, BPF, BFDGE and BADGE were 0.001, 0.002, 0.06, and 0.05 μg L−1, respectively. Finally, the method was applied to tap water, river water and snow water, the results show that the developed method is suitable for monitoring trace BPA, BPF, and their diglycidyl ethers in environmental water samples.
Introduction
Recently environmental security has been threatened by a wide range of chemical contaminants and has attracted great public attention. Various environmental chemicals may exert some harmful effects to animals and humans.1–3Bisphenol A (BPA) and F (BPF) are extensively used as leading chemicals in plastics with a variety of industrial applications, including food packaging, lacquer coatings in cans and dental composites and sealants. Bisphenol A diglycidyl ether (BADGE) and bisphenol F diglycidyl ether (BFDGE) are epoxy resins obtained by reaction of their respective monomers, BPA and BPF, with epichlorohydrin.4,5BPA exposure is the focus of a growing number of research studies, which has been mainly related to its estrogenic activity. The toxicity of BPF has been proven and it is mainly related to its estrogenic and antiandrogenic effect, while that of BADGE and BFDGE is related to their cytotoxic effects. The degree of toxicity of these compounds mainly depends on the fractional concentration of unreacted epoxy groups.6–10 Release of BPA, BPF, BADGE and BFDGE into the environment is possible during manufacturing and by leaching from final products. So, the determination of these compounds in environmental water samples is demanded for environmental risk assessment.The very low concentration level of these contaminants and the complex matrix of environmental water samples make the pretreatment usually necessary before instrumental analysis. In recent years, a number of studies have set forth various sample pretreatment techniques for these contaminants in environmental and food samples, including liquid–liquid extraction (LLE),8,11,12pressurized liquid extraction (PLE),13,14cloud point extraction,15 coacervative extraction,9solid-phase extraction (SPE),16solid-phase microextraction (SPME),17stir bar sorptive extraction (SBSE)18 and microextraction by packed sorbent (MEPS).19,20
Magnetic nanoparticles (MNPs) are nowadays one of the most important trends in science and applications including biotechnology and pharmacy fields as well as electronics, scientific tools, industrial manufacturing processes, the introduction of advanced materials, etc.21–26Carbon nanotubes (CNTs) have attracted considerable scientific and public interest in the past decade, regarded as promising candidates for reinforcement in composite materials due to their unique tubular structure and strong adsorption ability.27,28 It was found that CNTs reinforced composites have prominent enhancement in the properties, such as bending strength fracture toughness, wear resistance, corrosion resistance, electrical conductivity, and thermal stability. Therefore, these unique properties make CNTs very useful for supporting MNPs in many potential applications.29–38 Magnetic multiwalled carbon nanotube composites are hybrids of magnetite (Fe3O4) with multiwalled carbon nanotubes (MWCNTs). These composites combine the unique properties of MWCNTs and MNPs. MWCNT-MNP binding in the composite is strong enough to resist applied mechanical energy such as that of manual shaking or sonication. Such advantages show that magnetic CNTs may have great analytical potential as effective adsorbent for determination of some compounds.39,40 To the best of our knowledge, their analytical potential has been the subject of little research that has focused primarily on the development of synthetic methods and characterization of the resulting products.
In the present study, the preparation of magnetic MWCNTs and its application in the pretreatment of bisphenol-type compounds from environmental water samples is reported. Extraction conditions and derivatization conditions were optimized. Coupling with gas chromatography-tandem mass spectrometry (GC-MS/MS), a highly selective and sensitive analytical method was established. To our knowledge, our group demonstrated, for the first time, that magnetic MWCNTs are effective adsorbents for determination of BPA, BPF and their diglycidyl ethers in environmental water samples.
Experimental
Chemicals and materials
The standards of BPA, BPF, BADGE and BFDGE were purchased from Dr Ehrenstorfer (Augsburg, Germany). HPLC-grade methanol, n-hexane, acetonitrile and ethyl acetate were obtained from Tedia (Fairfield, Ohio, USA). Multiwalled carbon nanotubes (60–100 nm i.d.) were purchased from Nanotech Part Co., Ltd (Shenzhen, China). Analytical grade ethylene glycol, ferric chloride (FeCl3·6H2O), polyethylene glycol, ethanol, formic acid, aqueous ammonia and acetic anhydride were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Distilled water was purified by using Milli-Q ultra-pure water system (Milford, MA, USA).Three environmental water samples were evaluated including tap water, river water and snow water. Tap water samples were collected from Hunan Entry-Exit Inspection and Quarantine Bureau laboratory (Changsha, China) River water samples were collected from Xiang River (Changsha, China). All samples were collected randomly and filtered through 0.45 μm membranes to remove suspended particles. And snow water samples were collected from Yuelu Mountain (Changsha, China) and melted at room temperature in February 2011.
Standard solutions
Stock solutions of BPA, BPF, BADGE and BFDGE (1 mg ml−1) were prepared in methanol. These solutions were diluted daily to working concentrations with methanol. All solutions were stored at 4 °C in the dark.Derivatization of BPF, BPA standards
BPA, BPF and their diglycidyl ethers standard solution (100 μg L−1) was evaporated to dryness under nitrogen at 60 °C. 1 mL of 0.4 mol L−1Na2CO3 and 100 μL of acetic anhydride were added and the mixture was shaken for 30 s and allowed to stand for 15 min at 25 °C (room temperature). Afterwards n-hexane (2.5 mL × 2) was added, vortex for 1 min. After the organic phase and aqueous phase were separated thoroughly, the organic phase was transferred to a 5 mL glass centrifugal tube using a glass pipette. Then the combined n-hexane mixture was concentrated to 1.0 mL under a gentle nitrogen stream, which was ready for GC-MS/MS analysis.Preparation of the MWCNT-MNP composite
Our strategy to synthesize the MWCNT-MNP composite contains two main steps. (a) Functionalization of MWCNT: MWCNTs were treated with H2SO4/HNO3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ultrasonicated over 10 h to introduce hydroxyl and carboxyl groups onto their surfaces. (b) Assembling magnetic nanoparticles onto the acid-treated MWCNTs: An amount of 0.467 g of FeCl3·6H2O and 0.04 g acid-treated MWCNTs were suspended in 25 mL ethylene glycol in a glass vial. 1.2 g of sodium acetate was added and the solution was mixed by a magnetic stirrer at room temperature for 5 min. Afterwards the glass vial was placed in an airtight steel container and heated in an oven at 200 °C for 14 h. After cooling to room temperature, the synthetic product was washed with 25 mL ethanol and MWCNT-MNPs were recovered by applying a magnetic field via a magnet placed on the outer wall of the glass vial. This cleanup procedure was repeated 5 times. The MWCNT-MNP composite obtained was stored in ethanol (25 mL) until needed.
1) and ultrasonicated over 10 h to introduce hydroxyl and carboxyl groups onto their surfaces. (b) Assembling magnetic nanoparticles onto the acid-treated MWCNTs: An amount of 0.467 g of FeCl3·6H2O and 0.04 g acid-treated MWCNTs were suspended in 25 mL ethylene glycol in a glass vial. 1.2 g of sodium acetate was added and the solution was mixed by a magnetic stirrer at room temperature for 5 min. Afterwards the glass vial was placed in an airtight steel container and heated in an oven at 200 °C for 14 h. After cooling to room temperature, the synthetic product was washed with 25 mL ethanol and MWCNT-MNPs were recovered by applying a magnetic field via a magnet placed on the outer wall of the glass vial. This cleanup procedure was repeated 5 times. The MWCNT-MNP composite obtained was stored in ethanol (25 mL) until needed.
Extraction procedure
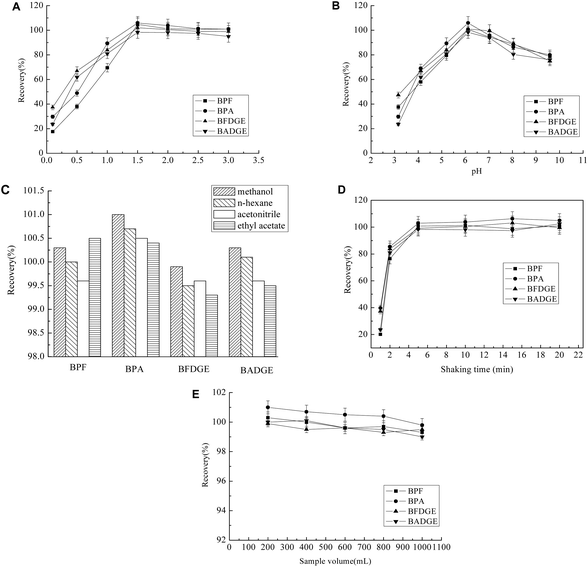
1.5 mg mL−1 MWCNT-MNP composite were added into 500 mL filtered water sample and the pH was adjusted to 6.1 with buffer. Then the mixture stand for 10 min after 5 min shaking. The supernatant was then removed and a magnet was paced on the outer wall of the vial in order to collect the MWCNT-MNP composite. Then the MWCNT-MNP composite was washed with 5 mL methanol that was subsequently removed using a pipette with the aid of the magnet again placed on the outer wall of the vial. Fig. 1 shows the extraction procedure. The final extract in methanol was transferred to the test tube and derivatized the same way as standard (2.2, described above). After derivatization, the sample was analyzed by GC-MS/MS. | ||
| Fig. 1 The MWCNT-MNP composite application for enriching analytes as sorbent. | ||
GC-MS/MS analysis
The analysis of all compounds was performed using a gas chromatograph (Varian-450) interfaced to a 300-MS triple quadrupole mass spectrometer (Varian Inc., USA), which contains data system software required for calibration, collection of GC-MS spectra and data processing for qualitative and quantitative analysis. Separation was achieved on a DB-1701 MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) from Varian Inc., USA. Ultra pure helium (99.999%) was used as a carrier gas at a flow rate of 1.0 mL min−1.1 μL of the concentrated extract was analyzed using splitless injection in Multiple Reaction Monitoring (MRM) mode. Injection port temperature was 250 °C and source temperature was 250 °C with electron energy of 70 eV. For the oven temperature program, an initial temperature was set up at 150 °C for 1 min, followed by an increase of temperature at the speed of 20 °C min−1 from 150 °C to 270 °C which was maintained for 28 min.
Results and discussion
Optimization of GC-MS/MS conditions
Identification of each compound was carried out by analysis of the compounds in the full scan mode. To optimize MS/MS conditions, after scanning and confirming the spectra using the NIST library search, the method was changed to the multiple reaction monitoring modes (MRM). Collision energies, dwell time and other parameters were optimized to improve the MS response for all the tested compounds. The most abundant Precursor and product ions were chosen for quantitation and the second for confirmation. A minimum of two transition ions were selected for each compound. The collision energies and other parameters used are shown in Table 1.| Compounds | RT (min) | Q1 Precursor ion (m/z) | Q3 Product ion (m/z) | Q/q a | Collision energy (eV) | Dwell time (s) | Q/q ratio b |
|---|---|---|---|---|---|---|---|
| a Q is quantification ion transition and q is confirmation ion transition. b Average value calculated from nine injections of standard solutions (three concentration levels replicates each). | |||||||
| BPF | 10.256 | 107 | 77 | Q | 15 | 0.2 | 3.27 |
| 200 | 107 | q | 10 | 0.2 | |||
| BPA | 11.010 | 213 | 119 | Q | 10 | 0.05 | 1.36 |
| 228 | 213 | q | 35 | 0.05 | |||
| BFDGE | 13.758 | 312 | 168 | Q | 15 | 0.1 | 1.93 |
| 181 | 152 | q | 25 | 0.1 | |||
| 16.407 | 312 | 168 | Q | 15 | 0.1 | 1.05 | |
| 197 | 141 | q | 25 | 0.1 | |||
| 20.213 | 312 | 168 | Q | 15 | 0.1 | 1.14 | |
| 312 | 198 | q | 15 | 0.1 | |||
| BADGE | 22.438 | 325 | 119 | Q | 25 | 0.1 | 4.75 |
| 340 | 269 | q | 20 | 0.1 |
With the help of the NIST standard mass spectral library, the characteristic ions and the retention times of the target compounds were obtained and identified with full scan mass spectra. Under the above optimized conditions, 6 peaks were obtained. In 25 min, all the target compounds could be well separated (shown in Fig. 2).
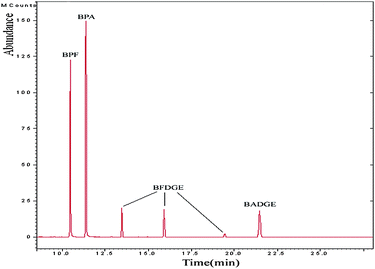 | ||
| Fig. 2 GC-MS/MS chromatogram of spiked water sample: BPA (0.1 μg L−1), BPF (0.1 μg L−1), BFDGE (2.0 μg L−1) and BADGE (2.0 μg L−1). | ||
Optimization of the derivatization conditions
BPA and BPF show poor peak shapes and sensitivity because of the hydroxyl polar group. Thus, their determination by GC–MS/MS requires a derivatization stage in order to minimize their polar character. Acetic anhydride has proved to be excellent derivatization agents for BPA and BPF, due to its advantages, such as simplicity or low-cost. Moreover, the process of derivatization can be carried out at room temperature in short times, which facilitate its application in routine analysis. During the derivatization reaction, the hydrogen atom from the –OH moiety is substituted by an acetyl group. Fig. 3 shows the schemes of derivatization reaction. | ||
| Fig. 3 The schemes of derivatization reaction. | ||
The method of derivatization was carried out based on previous methods described by Zhang et al..41 The standard solution volume containing the four analytes at 100 μg L−1 was used to optimize the derivatization step.
To investigate the effect of quantity of Na2CO3 on the performance of derivatization, various experiments were performed by adding different amounts of Na2CO3 (0.1–0.6 mol L−1) in sample solution. Other experimental conditions were kept constant. The results are shown in Fig. 4A. According to the results, 0.4 mol L−1Na2CO3 was chosen as the optimum concentration.
 | ||
| Fig. 4 Effect of (A) Na2CO3 concentration and (B) the volume of acetic anhydride on derivatization. | ||
To investigate the effect of derivatization reagent volume, several experiments were studied. Fig. 4B showed the curve of the recovery of BPA, BPF, BFDGE and BADGEversus volume of acetic anhydride. By increasing the volume of acetic anhydride up to 100 μL, the efficiency increased. Increasing acetic anhydride over 100 μL, caused a small decrease in efficiency. This decrease in the efficiency of the derivatization process might be a result of a raise in the acidity of solution (caused by hydrolysis of acetic anhydride).
The influence of reaction time was also examined. It was found that the derivatization was fast. The recovery was increased during the first 15 min, but no obvious changes were observed over 15 min. Therefore a reaction time of 15 min was selected.
Under the above optimized derivatization conditions, derivatized BPA, BPF present peaks with higher intensity and S/N ratios. For BFDGE and BADGE, the MS and chromatographic behaviors were affected slightly.
Characterization of the MWCNT-MNP composite
To confirm whether the product obtained was in fact the MWCNT-MNP composite, the material was characterized by a transmission electron microscope (TEM) of JEM-3010 (Jeol, Japan) with an accelerating voltage of 300 kV. For the preparation of TEM specimens, the MWCNT-MNP composite was dissolved in doubly distilled water and dropped onto a carbon-coated copper, as shown in Fig. 5. It was obvious that the nanotube surface was decorated by nanoparticles and no nanoparticles aggregation. The nanoparticles attached on the tube surface tightly and looked like nodes growing from the tubes. Such strong and complete attachment can be attributed to the cationic multiple arms grown on the tubes.42 Because the nanotubes were not completely covered by the MNPs, the composite was expected to have the sorption properties of MWCNTs and exhibit good ferromagnetic property of MNPs, which was sufficient for magnetic separation with a conventional magnet.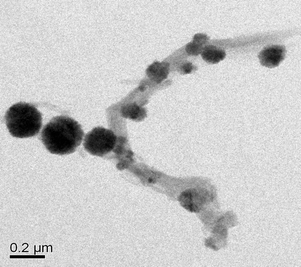 | ||
| Fig. 5 TEM images of the MWCNT-MNP composite. | ||
The FT-IR spectra of acid-treated MWCNTs and MWCNT-MNP composite are shown in Fig. 6. Some bands observed near 1580 cm−1 in samples showed the presence of the cylinder like carbon structure. The broad band around ca. 589 cm−1 was only visible in the spectra of MWCNT-MNP composite, proved that iron oxide existed in the composite. This was according to the work of Zuzana et al. and Jishi.43,44 Such results confirmed that MNPs were indeed coated onto the tube surface. This result is in good accordance with the TEM observation.
 | ||
| Fig. 6 FT-IR spectra of acid-treated MWCNTs and MWCNT-MNP composite. | ||
Optimization of the extraction conditions
Method validation
Linearity, limit of detection
Under the above optimized conditions, the calibration was constructed by plotting peak area against concentration. Quantitative parameters such as linear range, calibration equations, correlation coefficients and detection limits are summarized in Table 2. It suggests that the present method has wide linear range and satisfactory correlation coefficients (r2 > 0.995). The detection limits, which were calculated by using a signal-to-noise ratio of 3, for BPA, BPF, BFDGE and BADGE are 0.001, 0.002, 0.06 and 0.05 μg L−1, respectively.| Analytes | Linearity range (μg L−1) | Calibration equations | Correlation coefficients (r2) | Limits of detection (μg L−1) |
|---|---|---|---|---|
| BPF | 0.01–10 | Y = 630597x − 28635 | 0.9951 | 0.002 |
| BPA | 0.01–10 | Y = 548128x + 47912 | 0.9988 | 0.001 |
| BFDGE | 0.3–50 | Y = 643137x + 11769 | 0.9999 | 0.06 |
| BADGE | 0.1–50 | Y = 544221x + 64173 | 0.9992 | 0.05 |
Comparison of the present new method with the reported work
Comparisons with reported work are given in Table 3. This new method has the following merits: (a) After adsorption of analytes from water samples, the sorbents can be collected conveniently and rapidly with a magnet, which avoids the time-consuming column passing or filtration operation; (b) the magnetic multiwalled carbon nanotube sorbents possess high adsorption capacity and rapid adsorption rates, so a low amount of sorbent and short equilibrium time is required to extract analytes from large volumes of water samples; (c) higher recoveries and lower LODs were obtained for BPA, BPF, BADGE and BFDGE; (d) the GC-MS/MS method was developed which allows the confirmation of the presence of these compounds and the optimization of the detector settings, which has improved the limit of detection.| Instrument | The method of sample pretreatment | Analytes | Water sample (mL) | End volume (mL) | Recoveries (%) | LOD (μg L−1) |
|---|---|---|---|---|---|---|
| Gas chromatography-mass spectrometry [8] | Liquid-liquid extraction (80 g NaCl and laboratory-made equipment needed) | BPF | 500 | 0.2 | No data were reported | 0.02 |
| BPA | 0.006 | |||||
| BFDGE | 0.13 | |||||
| BADGE | 0.09 | |||||
| Liquid chromatography-fluorimetry [9] | Coacervative extraction (60 mg decanoic acid used) | BPF | 10.8 | 0.02 | 80–92 | 0.030–0.035 |
| BPA | ||||||
| BFDGE | ||||||
| BADGE | ||||||
| This work | MWCNT-MNP SPE | BPF | 500 | 1.0 | 88.5–115.1 | 0.002 |
| BPA | 0.001 | |||||
| BFDGE | 0.06 | |||||
| BADGE | 0.05 |
Precision, accuracy and recovery
The reproducibility of the method was determined by the intra-day and inter-day precisions. Five extraction of a mixture sample solution over a day gave the intra-day relative standard deviations (RSDs) and the inter-day precision was evaluated by extracting a mixture sample solution that has been independently prepared for three days continuously. It is shown in Table 4 that the results of the intra- and inter-assay showed good precision, with RSD values less than 10%.| Analytes | Intra-day precisions (RSD%, n = 5) | Inter-day precisions (RSD%, n = 5) | Recovery (%, n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 μg L−1 | 1 μg L−1 | 10 μg L−1 | 0.1 μg L−1 | 1 μg L−1 | 10 μg L−1 | 0.1 μg L−1 | 1 μg L−1 | 10 μg L−1 | |
| BPF | 1.5 | 5.6 | 2.9 | 5.0 | 4.1 | 1.7 | 115.1 ± 3.5 | 97.7 ± 2.5 | 100.6 ± 5.9 |
| BPA | 3.4 | 4.2 | 6.8 | 4.3 | 2.6 | 3.4 | 103.7 ± 4.0 | 98.4 ± 4.7 | 90.3 ± 2.3 |
| BFDGE | 2.8 | 3.2 | 6.5 | 3.5 | 4.7 | 8.0 | 99.7 ± 8.3 | 100.3 ± 7.1 | 101.3 ± 5.1 |
| BADGE | 5.9 | 4.4 | 5.6 | 4.5 | 5.6 | 2.9 | 88.5 ± 6.4 | 89.6 ± 1.8 | 102.4 ± 4.6 |
The recovery was measured by analyses of the spiked water samples at three different concentration levels (low, middle and high concentrations, the spiking levels ranged from 0.1 to 10 μg L−1) with five parallels at each level. Also as shown in Table 4, satisfactory recovery was obtained (88.5–115.1%) with the proposed method.
Application to real samples
In order to validate the suitability of the developed method, it was applied to detecting tap water and river water samples. Results are outlined in Table 5. The results show that BPA, BPF, BADGE, BFDGE were not detectable in tap and snow water samples. Meanwhile trace levels of bisphenol-type compounds were found in the river water samples (Fig. 8), with the recovery of 0.3 μg L−1 between 85.4–102.3%. Thus it was suitable for analyzing the water samples.| Analytes | Tap water | River water | Snow water | |||
|---|---|---|---|---|---|---|
| Detected (μg L−1) | Recoveries b (%) | Detected (μg L−1) | Recoveries (%) | Detected (μg L−1) | Recoveries b (%) | |
| a Mean of three determinations. b Standard deviation for three determinations. c Not detected. | ||||||
| BPF | ND c | 94.3 | 0.015 | 101.2 | ND | 98.2 |
| BPA | ND | 88.9 | 0.056 | 99.1 | ND | 102.3 |
| BFDGE | ND | 95.6 | 0.14 | 89.5 | ND | 99.4 |
| BADGE | ND | 100.1 | 0.35 | 96.4 | ND | 85.4 |
 | ||
| Fig. 8 GC-MS/MS chromatogram of river water sample. | ||
Conclusion
In this work, an MWCNT-MNP composite has been successfully prepared. A rapid, simple and efficient pretreatment of BPA, BPF, BFDGE and BADGE using the obtained material as a sorbent was accomplished based on magnetic separation. Compared with the reported methods, the use of MWCNT-MNP avoided the time-consuming column passing and filtration steps after the extractions, simplified sample preparation procedures, and showed great analytical potential in preconcentrating large volume water samples.The GC-MS/MS method was developed including MRM analysis of both quantitation and confirmation ion pairs which allow the confirmation of the presence of these compounds and the optimization of the detector settings had improved the limit of detection.
This new and confirmatory method was used to analyze BPA, BPF, BFDGE and BADGE in water samples and satisfactory results were obtained: High recovery (88.5–115.1%), low detection limits (0.001–0.05 μg L−1), and good repeatability (RSD < 10%).
All these results indicated that this study has proposed a rapid, convenient, low-cost, and harmless to the environment method to extract bisphenol-type compounds from water samples.
Acknowledgements
This work was jointly supported by National Key Technology program of China (No. 2011BAK10B05) and the National Natural Science Foundation of China (No 21175034).References
- O. Naoto, U. Yoko, Y. Satoru and K. Manabu, J. Health Sci., 2002, 48, 310–316 Search PubMed.
- J. Bernanke and H. R. Köhler, Rev. Environ. Contam. Toxicol., 2009, 198, 1–47 Search PubMed.
- B. Malene, F. R. Ulla, E. S. Niels and M. M. Katharina, European Journal of Endocrinology, 2005, 154, 599–611 Search PubMed.
- J. Lintschinger and W. Rauter, European Food Research and Technology, 2002, 11, 211–217 Search PubMed.
- J. Ivana, D. Jaroslav, V. Michal, P. Jan and J. Czech, Food Science, 2003, 21, 85–90 Search PubMed.
- N. B. Jonathan and R. Steintmetz, TEM, 1998, 9, 124–128 Search PubMed.
- P. P. Losada, C. P. Lamela, M. F. L. Fabal, P. S. Fenollera and J. S., J. Lozano, J. Agric. Food Chem., 1997, 45, 3493–3500 CrossRef.
- L. V. José, Z. Alberto, G. C. Antonio, E. Hontoria and O. Monsalud, Anal. Chim. Acta, 2001, 431, 31–40 CrossRef CAS.
- B. G. Ana, F. J. Ruiz, R. Soledad and P. B. Dolores, Anal. Chim. Acta, 2007, 603, 51–59 CrossRef CAS.
- P. Olga, Y. Vicent, L. Nuria and P. Agustín, J. Chromatogr., A, 2006, 1107, 70–78 CrossRef CAS.
- J. E. Biles, K. D. White, T. P. McNeal and T. H. Begley, J. Agric. Food Chem., 1997, 45, 3541 CrossRef CAS.
- J. S. Gándara, S. P. Abuin, P. L. Mahía, P. P. Losada and J. S. Lozano, Chromatographia, 1992, 34, 67–72 CAS.
- V. O. Yusà, P. M. Pardo and A. Pastor, Food Addit. Contam., 2005, 22, 482–9 Search PubMed.
- F. Emilia, S. Elisa, V. Sauro, F. Guillermina, M. Jordi and S. Gianni, Food Chemistry, 2011, 126, 1360–367 Search PubMed.
- Y. J. Yu, G. Y. Su, H. W. Michael, K. S. Paul and H. X. Yu, Chin. J. Anal. Chem., 2009, 37, 1717–1721 Search PubMed.
- X. H. Wu, L. Ding, Y. Zhang, X. X. Liu and L. B. Wang, Chinese Journal of Chromatography, 2010, 28, 1094–1098 Search PubMed.
- X. L. Cao and J. Corriveau, Journal of AOAC International, 2008, 91, 622–9 CAS.
- F. Lin, X. Huang, D. Yuan and B. Liu, Chinese Journal of Chromatography, 2010, 28, 507–12 Search PubMed.
- A. Prieto, S. Schrader and M. Moeder, J. Chromatogr., A, 2010, 1217, 6002–11 CrossRef CAS.
- A. Prieto, S. Schrader, C. Bauer and M. Möder, Anal. Chim. Acta, 2011, 685, 146–152 CrossRef CAS.
- S. X. Zhang, H. Y. Niu, Y. Q. Cai and Y. L. Shi, Anal. Chim. Acta, 2010, 665, 167–175 CrossRef CAS.
- L. Sun, C. Z. Zhang, L. G. Chen, J. Liu, H. Y. Jin, H. Y. Xu and L. Ding, Anal. Chim. Acta, 2009, 638, 162–168 CrossRef CAS.
- Y. Xiong, X. Xie, S. Chen and Z. Li, Chem.–Eur. J., 2003, 9, 4991–4996 CrossRef CAS.
- H. Y. Niu, Y. Q. Cai, Y. L. Shi, F. S. Wei, S. F. Mou and G. B. Jiang, J. Chromatogr., A, 2007, 1172, 113–120 CrossRef CAS.
- X. L. Zhao, Y. L. Shi, T. Wang, Y. Q. Cai and G. B. Jiang, J. Chromatogr., A, A 2008, 1188, 140–147 Search PubMed.
- J. H. Lin, Z. H. Wu and W. L. Tseng, Anal. Methods, 2010, 2, 1874–1879 RSC.
- G. Roberto, M. Monia, O. Guido, C. Johann, S. Tayyab, R. B. Aldo and S. Cristina, J. Mater. Chem., 2010, 20, 308–313 RSC.
- B. Suárez, B. M. Simonet, S. Cárdenas and M. Valcárcel, J. Chromatogr., A, 2007, 1159, 203–207 CrossRef CAS.
- P. Liang, Y. Liu, L. Guo, J. Zeng and H. J. Lu, J. Anal. At. Spectrom., 2004, 19, 1489–1492 RSC.
- S. Chakraborty, J. Chattopadhyay, H. Peng, Z. Y. Chen, A. Mukherjee, R. S. Arvidson, R. H. Hauge and W. E. Billups, J. Phys. Chem. B, B 2006, 110, 24812–24815 Search PubMed.
- D. W. Li, Y. T. Li, W. Song and Y. T. Long, Anal. Methods, 2010, 2, 837–843 RSC.
- F. Hennrich, R. Wellmann, S. S. Malik, Lebedkin and M. Kappes, Phys. Chem. Chem. Phys., 2003, 5, 178–183 RSC.
- P. Liang, Y. Liu and L. Guo, Spectrochim. Acta, Part B, 2005, 60, 125–129 CrossRef.
- Q. X. Zhou, J. P. Xiao and W. D. Wang, Microchim. Acta, 2007, 157, 93–98 CrossRef CAS.
- Y. Q. Cai, G. B. Jiang, J. F. Liu and Q. X. Zhou, Anal. Chem., 2003, 75, 2517–2521 CrossRef CAS.
- J. X. Wang, D. Q. Z. Y. Jiang, Gu and X. P. Yan, J. Chromatogr., A, 2006, 1137, 8–14 CrossRef CAS.
- N. Rastkari, R. Ahmadkhanih, M. Yunesian, L. J. Baleh and A. Mesdaghinia, Food Addit. Contam., Part A, 2010, 10, 1460–1468 Search PubMed.
- N. Rastkari, M. Yunesian and R. Ahmadkhaniha, Iran. J. Environ. Health. Sci. Eng., 2011, 8, 95–100 Search PubMed.
- C. G. Morales, A. Fekete, B. M. Simonet, R. Lehmann, S. Cárdenas, X. Zhang, M. Valcárcel and K. P. Schmitt, Anal. Chem., 2010, 82, 2743–52 CrossRef CAS.
- C. S. Chen, T. G. Liu, X. H. Chen, L. W. Lin, Q. C. Liu, Q. Xia and Z. W. Ning, Trans. Nonferrous Met. Soc. China, 2009, 19, 1567–1571 Search PubMed.
- J. Zhang, M. Gerard and H. A. Cooke, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2011, 879, 209–14 Search PubMed.
- C. Gao, W. Li, H. Morimoto, Y. Nagaoka and T. Maekawa, J. Phys. Chem. B, 2006, 110, 7213–20 CrossRef CAS.
- Zuzana MITRÓOVÁ, Natália TOMAŠOVIČOVÁ, Gábor LANCZ, Jozef KOVÁČ, Ivo VÁVRA and Peter KOPČANSKÝ, NANOCON, 2010, 10, 12–14 Search PubMed.
- R. A. JISHIl, Chem. Phys. Lett., 1993, 209, 77 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |