Capacitively coupled contactless conductivity detection on microfluidic systems—ten years of development†
Wendell Karlos Tomazelli
Coltro
ab,
Renato Sousa
Lima
bc,
Thiago Pinotti
Segato
bc,
Emanuel
Carrilho
bc,
Dosil Pereira
de Jesus
bd,
Claudimir Lucio
do Lago
be and
José Alberto Fracassi
da Silva
*bd
aInstituto de Química, Universidade Federal de Goiás, Campus Samambaia, 74001-970, Goiânia, GO, Brazil. E-mail: wendell@quimica.ufg.br; Fax: +55 62 3521-1127
bInstituto Nacional de Ciência e Tecnologia de Bioanalítica, Campinas, SP, Brazil
cInstituto de Química de São Carlos, Universidade de São Paulo, 13566-970, São Carlos, SP, Brazil. E-mail: emanuel@iqsc.usp.br
dInstituto de Química, Universidade Estadual de Campinas, 13083-970, Campinas, SP, Brazil. E-mail: fracassi@iqm.unicamp.br
eInstituto de Química, Universidade de São Paulo, 05508-900, São Paulo, SP, Brazil. E-mail: claudemi@iq.usp.br
First published on 7th October 2011
Abstract
The use of capacitively coupled contactless conductivity detection (C4D) on miniaturized systems has increased considerably over the last few years. Since the first report, 10 years ago, several advances on the detection cell geometry, strategies for increasing the sensitivity and a wide range of applications have been reported. This review intends to cover the main features related to the instrumental setup of this detection method for analytical and bioanalytical assays on microfluidic chips.
 Wendell Karlos Tomazelli Coltro | Wendell K. T. Coltro received his MS (2004) and PhD (2008) in Chemistry (Analytical Chemistry Concentration) from University of Sao Paulo. Since 2009, he has been an Assistant Professor at Institute of Chemistry from Federal University of Goias, Brazil. His research interests involve the development of alternative microfabrication technologies (toner-based technologies), coupling of electrochemical detectors and bioanalytical assays on microfluidic devices. |
 Thiago Pinotti Segato | Thiago P. Segato, PhD, is a postdoctoral student at the Institute of Chemistry at Sao Carlos, Brazil, and an associate researcher at the INCTBio. His current research is focused on fabrication of microfluidic chips and their coupling with detection techniques. |
 Emanuel Carrilho | Emanuel Carrilho is an Associate Professor in Bioanalytical Chemistry at the University of São Paulo, Brazil. He obtained his PhD in Bioanalytical Chemistry at the Barnett Institute, Northeastern University and took a two-year sabbatical at Harvard University. His current research interests focus on the development of analytical methods for biological systems and the development of instrumentation based on microfluidics for diagnosis of disease. |
 Dosil Pereira de Jesus | Dosil P. Jesus received his MS (1999) and PhD (2003) in Analytical Chemistry from the Institute of Chemistry at the University of São Paulo (Brazil), where he was a postdoctoral research fellow (2004–2005). From 2009 to 2010 he was a visiting scholar at the Department of Chemistry and Chemical Biology at Harvard University. He is currently a professor in the Institute of Chemistry at State University of Campinas (Brazil). His research is focused on capillary electrophoresis separations and development of microfluidic devices for Analytical Chemistry. |
 José Alberto Fracassi da Silva | José Alberto Fracassi da Silva graduated in Chemistry from the São Paulo State University in 1996, where he also received his PhD in Analytical Chemistry in 2001. Afterwards, he got a post-doctoral position at the Laboratory of Integrated Systems, in Polytechnic School in the University of São Paulo. In 2004, he got a position at State University of Campinas, in Campinas, SP, Brazil. In 2010 he was a visiting scholar at The Ralph Adams Institute for Bioanalytical Chemistry, The University of Kansas (USA). His main research interests are focused on instrumentation and methods for both capillary and microchip electrophoresis. |
Introduction
The use of microfluidic platforms for chemical and biochemical applications has achieved remarkable progress in the last two decades.1–4 The miniaturization of analytical components (valves, pumps, mixers, pH regulators, reactors, etc.), the development of ultrasensitive methods, and the integration of different detectors with separation techniques are attractive features in this field.1,2 Although a rapid advance has been observed in this area, greater efforts should be spent to fabricate and use real micro total analysis systems (μTAS).5 These devices, which were conceptually designed in 1990 by Manz et al.,6 involve the on-chip integration of multiple analytical steps including sampling, sample pretreatment, separation, chemical reactions, detection, and even electronics for data acquisition. This characteristic is highly relevant in many cases, such as in clinical diagnostics and health care.7Over the past years, different detection systems, including fluorescence,8mass spectrometry,9 and electrochemical detectors,10–12 have been coupled with microfluidic systems. Due to its inherent compatibility with miniaturization techniques, electrochemical detection is becoming increasingly popular for on-chip applications.10–15 By using standard10–15 or alternative16 microfabrication technologies, detection electrodes and microfluidic channels can be integrated and co-fabricated on a single device. Among all electrochemical techniques, the capacitively coupled contactless conductivity detection (C4D) is one of the most promising for microfluidic applications.13–15
The C4D history started in 1928, when Zahn17 reported that conductance measurements could be carried out without physical contact of the electrodes with the measured solution, as reported by Burkhalter.18 The contactless conductivity technique was popular in the 1960s under the format of “High Frequency Titrations” but its use was discontinued in the next decades, mainly due to advances in the instrumentation and methods for chromatography. A new impulse occurred in the 1980s when “Oscillometry” was employed as a detection scheme for flow injection analysis and chromatography.19–21 A few reports about C4D applied to isotachophoresis appeared during the 1980s, being the first reports for electroseparation techniques.22–24 In 1998, the use of C4D coupled to capillary electrophoresis (CE) was proposed independently by Zemann et al.25 and by da Silva and do Lago.26 Further information about the fundamentals, instrumentation and applications using conventional CE-C4D can be found in excellent review articles.14,15,27–32
The first report about C4D on microfluidic systems was published in 2001, by Guijt et al.33 The use of a contactless electrochemical detection mode offers some advantages over faradaic electrochemical methods. The electrical insulation of the electrodes from the electrolytic fluid avoids problems commonly found in the contact mode including electrode fouling, bubble formation (electrolysis), and electrical interference due to the electric field applied in electrophoretic systems, for example.13–15,27–33 The main disadvantage of the C4D system is attributed to its lower sensitivity when compared to other electrochemical modes of detection, e.g., to amperometric32 or contact conductivity31,34 detection.
Since the first publication, many reports on microchips with C4D have appeared in the literature. In 2007, Pumera reported an outstanding review article exclusively dedicated to the designs and applications of C4D on microfluidics.13 This current review describes the main advances about C4D on microfluidic systems reported in the last ten years. The detection cell geometry, the strategies for increasing the sensitivity as well as the main applications are presented and discussed.
Theory of C4D
In C4D, the faradaic current is absent, because the electrodes are insulated from the solution by a dielectric and, then, we say that they are capacitively coupled to the electrolyte. On microchips, the electrode/dielectric/solution system exhibits behaviour similar to a parallel plate capacitor.In direct conductivity, a small amplitude alternate signal (a sinusoidal wave, for example) is applied to two inert electrodes as illustrated in Fig. 1(a). Since the electrodes are in direct contact with the solution, a low frequency is used since the capacitance of the electrical double layer (EDL) is relatively high, approximately a few μF per cm2.35 The use of an alternate signal also minimizes the electrolysis effects on the conductivity measurement. If the electrodes were placed outside the reservoir (Fig. 1(b)), the capacitance of the wall of the reservoir will impose a high impedance to signal transmission or, more accurately, a high capacitive reactance (XC). XC is inversely proportional to both the operating frequency and the capacitance value—in this case, the capacitance of the wall of the reservoir (Cw). Since Cw is very small, a high frequency signal must be applied to the electrodes to overcome the capacitive reactance of the reservoir.
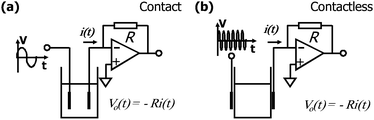 | ||
| Fig. 1 Comparison between (a) contact and (b) contactless conductivity detection. The current i(t) is proportional to the conductance of the solution, and this information can be coded as a voltage Vo(t) by using a current-to-voltage converter (also known as transimpedance amplifier). | ||
Fig. 2 illustrates a typical C4D microchip and the simplest equivalent electrical circuit26,36 is composed by the following elements: (i) solution resistance (RS); (ii) capacitances relative to the dielectric, defined by the electrode geometry and wall thickness (Cw); and (iii) a third capacitance resulting from the direct capacitive coupling between the electrodes, called stray capacitance (C0). In general, the C4D consists in applying an alternating signal to a detection cell electrode (excitation electrode, eexc), which generates the polarization of the dielectric. This phenomenon induces ion movement in the electrolyte solution due to the formation of an interfacial potential difference. Finally, in the region of the second electrode (receiver electrode, er), an analogous process occurs resulting in an alternate current between the electrodes.
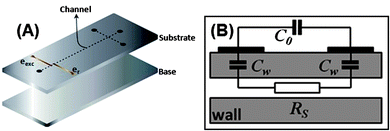 | ||
| Fig. 2 Representation of (A) constituent layers of a microfluidic system with a typical C4D microcell and (B) its equivalent electrical circuit. In (A), the substrate contains microchannels and electrodes in its bottom and top surfaces, respectively. Cw, C0, and RS stand for the wall capacitance, stray capacitance, and solution resistance, respectively. | ||
The current measured by the C4D detector reflects the system total admittance (the inverse of the impedance), which depends on the capacitive reactance of the dielectric wall (XCw), the resistance of the solution (RS), and the reactance of the stray capacitance (XC0).
When simultaneously XCw is made low and XC0 is made high, one can infer that the cell admittance (and, thus, the resulting current or voltage signal) varies linearly with the conductivity of the solution. However, for the typical configurations used for C4D, the presence of the capacitances leads to non-linear behaviour of the cell admittance as a function of the conductivity of the solution. Fortunately, the analytical signal represents only a very small variation of conductivity; so small that one can approximate a straight line to that region of the curve (the reader can have an insight about this discussion by considering the concept of mathematical differentiation, i.e., under a close look, any curve segment can be approximated to a straight line).
It is also important to highlight that conductivity is a universal method of detection, since the response will be promoted by any ion in solution. The method requires a separation step in order to enhance the selectivity. This is the reason for the success of conductivity-based detectors for chromatography and electroseparation techniques.
Detection cell geometry
Since the first reports,33,37C4D has been extensively applied to monitor electrophoretic separations, chemical reactions, as well as bioassays on microchips. One of the main trends is its coupling with microchip electrophoresis (MCE). When compared to the C4D instrumentation employed for conventional CE, the most remarkable difference is the electrode format used for MCE. In fused-silica capillary, tubular electrodes are placed around the outer polyimide coating of the capillary. The integration of this geometry on miniaturized devices is not compatible with the main fabrication technologies available today. Thus, planar electrodes, which are much easier to be integrated into the chip-based devices, are nearly the only geometry.13,14Regarding the detection cell arrangement in MCE, basically the electrodes may be (i) embedded in the microchip, (ii) attached to it (placed either underneath the bottom or on top of the device) or (iii) can be external to the device (see Fig. 3). In the first option, the electrodes are physically separated from the inside microchannel with an insulating layer to enable the capacitive coupling. In the second one, the bottom plate or top cover thicknesses provide insulation from the microfluidic channel. The last option is similar to the second one, but the electrodes are not part of the device, i.e., they are made on a holder and are preserved when the fluidic device is discarded. The significant thickness of the top and bottom parts causes an augment of the impedance of the wall. On the other hand, the external electrodes are advantageous, because they are not part of the microchip itself.13,14
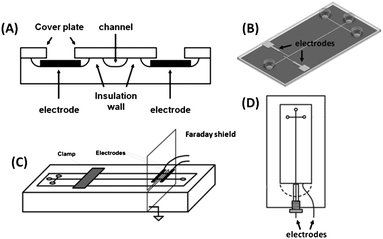 | ||
| Fig. 3 Detection cell geometries for MCE-C4D. (A) Representation of in-plane embedded electrodes; (B) design of attached electrodes directly on MCE; (C) scheme of an external holder containing two electrodes and their coupling with MCE; and (D) wall-jet configuration on the chip. (A), (B), (C), and (D) were adapted with permission from ref. 38–41, respectively. | ||
The electrodes for C4D measurements on MCE (MCE-C4D) have been fabricated by different approaches including photolithographic steps, screen-printing techniques, metal sputtering or evaporation, as well as using practical and inexpensive tools like silver paint, aluminium foil, adhesive metal tapes, or printed circuit boards (PCB). The following sections will present a short review about the improvements in detection cell geometry and analytical sensitivity as well as recent applications using C4D on microfluidic devices.
Geometry of the detection electrodes
As mentioned before, the electrodes for MCE-C4D can be embedded (Fig. 3(A)), attached (Fig. 3(B)), or external (Fig. 3C and D) to the microfluidic device. Due to compatibility with microfabrication techniques, electrodes and channels can be incorporated on the same substrate. This compatibility allowed constructing microsystems containing channels and planar electrodes for electrochemical detection. Regarding C4D measurements, electrodes have been often insulated from the separation channel by using thin film deposition technologies. However, simpler and easier approaches have also been reported.Embedded electrodes
The first report about MCE-C4D was published by Guijt et al.33 In this work, a four-electrode configuration was fabricated in a glass substrate and insulated by a 30 nm thick silicon carbide layer. The wafers containing the electrodes and etched channels were integrated by anodic bonding at 400 °C and 100 V for 1 h.33,42 Berthold et al. reported a very similar approach.37Lichtenberg et al. reported a new approach to integrate in-plane electrodes for C4D with glass channels.38 Microchannels and electrodes were formed on glass wafers in sequential photolithographic steps. Platinum electrodes were fabricated by first evaporating 20 nm of tantalum as adhesion layer, followed by 150 nm of Pt. A thin glass wall of 10–15 μm (see Fig. 3(A)) provided the separation between the detection electrodes and the buffer electrolyte. The wafer containing the etched-glass channels and the electrodes was fused against another glass plate by thermal bonding at 650 °C.
Lee et al. reported the construction of semicircular electrodes for C4D on a glass chip.43 The electrodes were deposited around the separation channel by Cr/Au sputtering and patterned using the lift-off process as depicted in Fig. 4. The electrodes were separated from the microfluidic channel at a distance of 40 μm. Although the use of semicircular detection electrodes seems to provide better sensitivity, the authors did not supply an explanation for the shift of the operation frequency to the range of 5–25 kHz, which is unusual for C4D. Also, it is not very clear why the resolution is better using the circular geometry and why the migration order, in some electropherograms, for common cations in 10 mmol L−1MES/His does not agree with previous published papers.
 | ||
| Fig. 4 Scanning electron micrographs showing (A) the detection section of the separation channel, and (B) semicircular and (C) planar electrodes in the bonded chips. Reprinted from ref. 43, with permission. | ||
Shang et al. reported the fabrication of a MCE-C4D with insulated detection electrodes.44Chromium electrodes were formed using an e-beam evaporator and a 100 nm SiO2 layer provided the electrical insulation. The insulating layer was deposited by plasma-enhanced chemical vapour deposition (PECVD).
In 2010 and 2011, some authors have reported simpler methods to fabricate embedded electrodes for performing MCE-C4D. Segato et al. explored the use of a thin PDMS layer (50 μm thick) to provide the electrical insulation of glass channels to sputtered electrodes (see Fig. 5). This PDMS membrane enabled the capacitive coupling, and also allowed the sealing of the glass channels at room temperature.45 Liu et al. have also explored the use of a PDMS membrane to insulate electrodes fabricated on glass substrates and to enable the capacitive coupling for C4D.46 A similar strategy was adopted to produce a three-layer PMMA MCE, in which a 50 μm layer provided the insulation of Pt microelectrodes.47
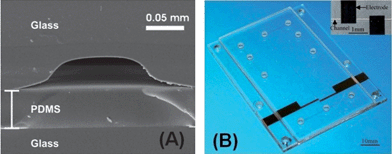 | ||
| Fig. 5 Examples of (A) a SEM image from the transversal section of the glass channel sealed by a thin PDMS layer, and (B) optical micrograph of a three layer PMMA device with integrated Pt electrodes for C4D. Figures (A) and (B) were reprinted, with permission, from ref. 45 and 47, respectively. | ||
Coltro et al. have reported a simple approach to produce polymeric microchips with integrated copper electrodes for C4D.48Copper electrodes were fabricated using a printed circuit board (PCB), as an inexpensive source of thin metal film, and toner masks, for the wet chemical etching of the metal layer on the PCB. A poly(ethylene terephthalate) (PET) film coated with a thin thermo-sensitive adhesive layer was laminated to the PCB plate, providing the insulation of the electrodes to perform C4D measurements. Guijt et al. have also demonstrated the photolithographic fabrication of planar electrodes on PCB substrates for C4D on MCE.49
Attachment of electrodes
One of the limitations of using embedded electrodes is the dependence on sophisticated instrumentation to provide an effective and reproducible insulation between the solution channel and the electrode. A non-effective electrical insulation between the electrodes and the microfluidic channel can lead to a damage of the detector's electronic circuit, due to the high electric fields used in the separations. In practice, it is very common to find defects on the insulation layer when the thickness is around tens of nm. For this reason, the search for effective and simpler configurations has been the focus of many research groups.In 2002, Pumera et al. described one of the simplest methods to couple C4D electrodes with MCE.50 Two rectangular-shaped electrodes, fabricated from aluminium strips, were placed on the outer side of PMMA microfluidic chips. In this case, the substrate thickness (125 μm) provided the insulation between the electrodes and the solution. Due to its simplicity and low instrumental requirements, this approach has been adopted by other groups. This system was adapted, by Pumera and Wang,51 to perform dual amperometric and C4D measurements on MCE.
Tanyanyiwa and Hauser,52 at the end of 2002, published the use of high-voltage C4D for MCE. In their report, the application of an AC excitation signal of 500 Vpp and a frequency of 100 kHz allowed them to detect through a cover plate of 1 mm thickness. As detection electrodes, the authors used two parallel strips 0.5 mm wide and 5 mm long that were formed with silver paint.
A movable C4D system was adapted from the conventional CE to MCE system.53,54 Rectangular shaped electrodes were fabricated from 10 μm thick aluminium foil and glued around two sides of the 1 mm thick PMMA plate of the detector. The cover plate of the chip (125 μm thick) was thus mechanically pressed against the detection electrodes. The position of the electrodes could be changed by “sliding” the detector holder along the separation channel.
In 2003, do Lago et al. demonstrated the coupling of C4D with polyester-toner (PT) devices.55Electrodes were formed using adhesive copper strips that were glued outside the polyester film. The 100 μm thickness allowed capacitive coupling with the solution. This same approach was later used for glass-toner devices.56 Ding et al. also showed that tapered rectangular electrodes for C4D can be attached to PDMS chips.57 Wang et al. demonstrated the use of the screen-printing process for generating highly reproducible sets of silver-ink electrodes for MCE-C4D.58 Printing of silver electrodes was performed through a patterned stencil on thin PMMA sheets. The screen-printed PMMA sheets were cut, aligned and sealed to the channel plate thus establishing a complete MCE-C4D.
The integration of planar electrodes on PT devices was also demonstrated by Coltro et al.39 Aluminium electrodes were sputtered on polyester films using toner masks that defined the electrode area (see Fig. 3(B)). After removal of the toner, the polyester with incorporated electrodes (on the outer surface) was laminated, at 40 cm min−1 and 120 °C, against a printed PT channel layer (on the inner surface) to create a low cost MCE-C4D on PT with integrated electrodes.
Most recently, an end-to-end differential MCE-C4D was reported by Fercher et al.59 This new approach is based on the placement of two distinct detector areas (consisting of two screen-printed silver electrodes) near the ends of the fluid inlet and fluid outlet of the separation channel. Both output signals are subtracted from each other, and the resulting differential signal is amplified and measured. This measurement approach has several advantages over the established single-end detection. The high baseline level resulting from parasitic stray capacitance and buffer conductivity is reduced, leading to better signal-to-noise ratio (S/N) and hence higher sensitivity. Furthermore, temperature and, thus, baseline drift effects are diminished owing to the differentiating nature of the system. These last two configurations will be better discussed ahead.
External electrodes
Tanyanyiwa et al. described the use of external electrodes on a holder to perform high voltage C4D (HVC4D) on MCE.40 In this approach, two electrodes, fabricated by using strips of adhesive copper tape, were mounted on a holder constructed from PMMA (see design presented in Fig. 3(C)). The holder also features a vertical Faraday shield, which is employed to minimize direct capacitive coupling between the electrodes. For operation, the chip is placed and then clamped onto its holder. This instrumentation is very simple and allows the use of any kind of substrate material.Chen et al. reported the coupling of a glass microchip,60 bonded with a thin cover glass plate, with an external copper plate containing detection electrodes previously fabricated by wet chemical etching. The authors investigated the effect of sine, square, and triangular waveforms. The sine wave offered the most favourable S/N. This conclusion agrees with results previously published by Wang et al.61
Wang et al. also developed a “hybrid” electrode configuration for performing MCE-C4D.41 It is based on an arrangement where the receiving electrode is insulated by a thin layer of poly(vinylchloride) and placed in the bulk solution of the detection reservoir of the chip, whereas the emitting electrode is in contact with the solution eluted from the channel outlet in a wall-jet configuration (see design depicted in Fig. 3(D)).
Recently, Mahabadi et al. described a dual top–bottom C4D cell configuration for microchips.62 The sensing cell consists of two pairs of copper strips embedded into two polymer blocks and placed inside the housing at the top and the bottom. When compared to conventional top–top electrode geometry, the dual top–bottom geometry exhibited improved detector performance.
Alternatives for increasing the C4D sensitivity
The main disadvantage reported for the C4D systems is their lower sensitivity when compared with other electrochemical detection methods. This low sensitivity arises from two phenomena: absence of charge transfer on the electrode surface and direct capacitive coupling between the electrodes, generating the stray capacitance. The alternatives described in the literature in order to improve the C4D sensitivity are based on three strategies, namely: (i) increasing the detection area, (ii) decreasing the dielectric thickness, and (iii) minimizing the stray capacitance. The hybrid detection mode,41 containing the emitting electrode in contact with the electrolyte, can be included in the second case. Such arrangement provided over 10-fold sensitivity enhancement compared to the full wall-jet contactless conductivity detector.According to eqn (1), the detection area (A) and the dielectric thickness (d) affect the capacitance (C) of the electrode/dielectric/solution system. Consequently, the C4D signal is also affected.
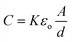 | (1) |
The stray capacitance (C0), by its turn, occurs owing to direct capacitive coupling between the electrodes, which can cause detection problems due to the drop of the equivalent impedance of the detection cell.26,28,29 Stray capacitances can affect the peak height, increase the background signal, and generate peak overshooting leading to a deterioration of the S/N. This phenomenon is particularly pronounced at higher frequencies.63 Some studies about the improvements on sensitivity are presented in the following sections.
Detection area
The approaches aiming at increasing the sensing area of integrated electrodes include: dual top–bottom,62 semicircular,43 and sidewall electrodes. These last two configurations consist of embedded electrodes placed on the same plane of the microchannel and with greater detection area. The dual top–bottom electrode geometry, in conjunction with thin electrophoresis microchips, doubles the total wall capacitance and enables an enhancement on the signal coupling with the detection volume. Compared to the top–top geometry, this configuration gave an increase in signal ranging from 40 to 65% in terms of peak height for NH4+ and Li+, respectively. The reported data in this case represent the best limits of detection for small inorganic ions found in the literature, with a detection limit of approximately 0.3 μmol L−1 for cations and 0.15 μmol L−1 for anions.62Thickness of the dielectric layer
Besides the detection area, the thickness of the dielectric layer also directly affects the C4D sensitivity. As reported by Kubáň and Hauser,64 there is a loss of approximately 75% of the analytical signal when this thickness is increased from 125 to 425 μm, with C4D electrodes in antiparallel orientation (see Fig. 6(A)). In general, the minimum thickness values found for C4D devices range between 0.1 and 40 μm for embedded electrodes and between 100 and 200 μm for both attached and external electrodes. Regarding the embedded electrodes configuration, Guijt et al. depicted the insulation with SiC thin films of approximately 30 nm thickness.33 The proposed method, however, requires a laborious fabrication process and exhibits limited electric field stability. Presumably, the limited stability problem occurred as a function of a non-effective insulation of the electrodes or the dielectric strength. | ||
| Fig. 6 (A) Schematic drawing of detection electrodes in (i) parallel and (ii) antiparallel orientation. (B) Effect of the parallel (square) and antiparallel (circle) design of electrodes on output voltage (solid), peak heights (open, dotted line) and S/N (open, solid line) for the lithium peak. Reprinted from ref. 64, with permission. | ||
Stray capacitance
The orientation of the electrodes and the use of Faraday shield (see Fig. 3(C)) list as alternatives for reduction of the C0 value. In 2005, Kubáň and Hauser64 investigated the effects of cell geometry and operating parameters on the performance of external electrodes for C4D on MCE. One of the evaluated parameters was the orientation of the electrodes: parallel or antiparallel, as shown in Fig. 6(A). In this study, the cell output voltage, the peak heights, and the S/N were evaluated as functions of the excitation frequency. Due to the higher stray capacitance, the parallel orientation was found to give higher output voltage. As expected, the greater stray capacitance results in a reduced peak height and also lower S/N as shown in Fig. 6(B). Electrodes in antiparallel orientation have been often adopted for C4D measurements on MCE owing to their better sensitivity.Finally, reports discussing other parameters that also affect the C4D sensitivity—including the cell geometry configuration, electrode dimensions (length, width, and gap) and electronics—can also be found in the literature.64–66
Applications
The coupling of C4D on microfluidic systems has led to a large range of applications. Some examples including bioanalytical assays, on-chip enzymatic reactions, food analysis, and determinations of explosives, and chemical warfare agents are listed below.Bioanalytical applications
MCE-C4D has been applied for the determination of a wide range of biochemical species including amino acids, proteins, peptides, DNA, and immunoglobulin G (IgG). Tanyanyiwa et al. showed the first separation and detection of amino acids on MCE using C4D.40,67 One year later, Abad-Villar et al. reported the detection of a wide range of biochemically relevant species.68 In this study, 12 essential amino acids were detected in approximately 4 min using a 2.3 mol L−1acetic acid solution (pH 2.1) as BGE. The separation of peptides (leucine enkephalin, bradykinin, angiotensin II, thyrotropin releasing hormone, and oxytocin) was also demonstrated using the same media with addition of 0.05% Tween 20. Electrophoretic separations of the proteins cytochrome c, human serum albumin (HSA), and myoglobin were also shown. For the applications mentioned here, separation efficiencies up to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plates could be obtained and LODs were in the low μmol L−1 range, except for IgG that could be determined down to 0.4 nmol L−1.
000 plates could be obtained and LODs were in the low μmol L−1 range, except for IgG that could be determined down to 0.4 nmol L−1.
Analytical feasibility has been also shown for detecting neurotransmitters (dopamine, ephedrine, and metanephrine), β-blockers (pindolol, atenolol, and acebutolol) and some physiologically active amines (doxylamine, octopamine, noradrenaline, adrenaline, and isoproterenol) using MCE-C4D.67,69
Gong and Hauser70 reported the baseline separation of the enantiomers of trans-cyclohexane-1,2-diamine is less than 2 min. In this case, MCE with C4D was quite advantageous since these compounds have neither strong UV-absorbance nor fluorescence. The determination of 3-methylhistidine (3-MH) and 1-methylhistidine (1-MH) on MCE-C4D was reported by Tuma et al., in which a silicon microchip with electrodes made of aluminium strips was employed.71 Both compounds are of clinical relevance. Kubáň and Hauser showed recently the determination of inorganic ions in clinical samples (serum and urine samples) using MCE-C4D.65 Bare electrophoresis chips were used in combination with external electrodes.
On-chip enzymatic reactions
Wang et al. described an on-chip enzymatic assay for screening organophosphate (OP) nerve agents,72 based on a pre-column reaction of organophosphorus hydrolase (OPH) and electrophoretic separation of the phosphonic acid products by MCE-C4D. The complete bioassay was carried out in around 3 min including the OPH reaction, separation and detection of the reaction products.Abad-Villar et al. performed on-chip enzymatic digestions of HSA using pepsin.68 As the enzymatic digestion proceeds, the amount of different fragments varies, resulting in changes in the peak heights and shapes. The products of the enzymatic reaction were successfully monitored with MCE-C4D. Schuchert-Shi et al. successfully showed the conversion of glucose to gluconate using glucose oxidase and the enzymatic hydrolysis of ethyl acetate generating acetate ions.73 These same authors reported one year later the monitoring of the production of ammonium from urea in serum samples.74
Vázquez et al. have demonstrated the ability of MCE-C4D to monitor the detection ofperoxynitrite degradation products.75Peroxynitrite (ONOO−) is a strong oxidizing agent that is produced in cells from the reaction of superoxide (O2−) and nitric oxide (NO˙). It degrades rapidly into its main metabolites: nitrate and nitrite. Both metabolites were successfully detected on MCE by C4D and amperometric detection coupled in-line.
Food analysis
A quantitative analysis of inorganic and organic ions in alcoholic (beer and wine) and non-alcoholic (potable water, fruit juices and milk) beverages has been performed by MCE-C4D.76 Law et al. reported the application of MCE with C4D to determine food additives in soft drinks and vitamin C tablets.77 For the vitamin C tablet, besides the ascorbate (that is the major component) small amounts of chloride, citrate, maleate, and acetate were also found. For the soft drinks, sorbate and benzoate are the major additives present, accompanied by very small amounts of acetate and lactate.Lee et al. reported the determination of major inorganic cations in commercial sports drinks, mineral waters, and red wine.43 Mühlberger et al. also reported an analytical method to separate carbohydrates on MCE-C4D.78 Some carbohydrates such as glucose, fructose, and sucrose were determined in fruit nectar, fruit juice, and cola samples using polyether ether ketone (PEEK) chips.
Lu et al. reported that the rapid and quantitative determination of fluoroacetate in diluted fruit juices and tap water was performed by MCE-C4D.79
In 2011, Mark et al. reported a method for the efficient extraction and determination of volatile aliphatic amines in the biodegradation process of seafood samples.80 The authors combined single-drop microextraction (SDME) with MCE-C4D. The separation and detection of methylamine, dimethylamine, trimethylamine, diethylamine, and triethylamine was performed in less than 40 s. The LODs were lower than 400 ng mL−1.
Explosives and chemical warfare agents
MCE-C4D appears to be a very promising tool to detect major explosive-related ions (ammonium, methylammonium, potassium, sodium, perchlorate, chloride and nitrate) as well as their residues at the sites of terrorist attacks. Wang et al. described the use of MCE-C4D for the rapid separation and detection of explosive-related cations and anions using the same microchannel and running buffer.81 Using a movable C4D and a dual-opposite injection strategy, the separation/detection of these explosive-related ions was also demonstrated.41,53,54Nerve agents derive their name from their adverse effect on the nervous system. In response to recent terrorist activity and to the ratification of the Chemical Warfare Convention, there are urgent demands for rapid and reliable methods for the determination of chemical warfare agents (CWAs) and their degradation products.81 Wang et al. demonstrated the application of MCE-C4D for the screening of organophosphonate nerve agent degradation products.61 The miniaturized system relies on an efficient chip-based separation of alkyl methylphosphonic acids (hydrolysis products of sarin, soman, and VX nerve agents). The applicability to analysis of natural (river) water samples was demonstrated. Wang et al. demonstrated the same application using a movable C4D.53 Nerve agents were also used as model analytes to evaluate a MCE with dual-opposite injection strategy.54 Ding et al. have also shown the applicability of a PDMS device with C4D for the determination of organophosphonate nerve agent degradation products.57 Applicability of this method to natural (lake and tap) water samples was also demonstrated.
Ding and Rogers reported the use of MCE-C4D for the determination of nitrogen mustard gas degradation products.82 Three alkyl ethanolamines: N-methyldiethanolamine (MDEA), N-ethyldiethanolamine (EDEA), and triethanolamine (TEA), (degradation/precursor products of HN-1, HN-2 and HN-3 blister agents) were analyzed by MCE-C4D. The original PDMS channel was coated with poly(ethyleneimine) (PEI) to improve the separation of the three ethanolamines.
Other applications
Tanyanyiwa et al. reported the separation of sucrose and fructose67 using a 10 mmol L−1sodium hydroxide electrolyte or a 100 mmol L−1triethylamine buffer at pH 12. Better sensitivity was obtained with the sodium hydroxide electrolyte. Chen et al. used a movable C4D to detect sodium and chloride ions in seawater samples.83 In addition, detection of organic acids and pharmaceutical substances67 as well as heavy metals40 has also been described by other groups using MCE-C4D.Walsh et al. reported the use of scanning C4D (sC4D) for the evaluation of the structural homogeneity and density of both packed and monolithic stationary phases in microfluidic chips.84 The chip was placed on top of the substrate containing the copper electrodes and, by sliding the chip through the microchannel, the packed columns were sequentially scanned along their whole length, from the beginning to the end as illustrated in Fig. 7(A). In order to continue scanning another channel, the chip was simply displaced accordingly to the left or right. The resulting sC4D profiles are shown in Fig. 7(B). As can be seen, there are dramatic changes in the C4D response observed for packed and unpacked regions.
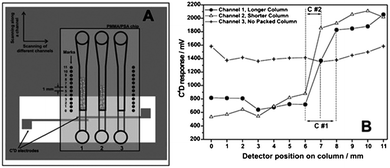 | ||
| Fig. 7 Representation of the (A) measurement setup for sC4D and (B) typical scan results for packed microfluidic channels. Reprinted from ref. 84, with permission. | ||
Conclusions and future trends
The implementation of C4D on MCE fabricated with different substrate materials with conventional and alternative microfabrication techniques was described. Since UV-vis transparent window detection is not required in C4D, microchips made with materials other than glass can be used. In addition, as the electrodes used in the detection are not in contact with the solutions, inexpensive materials (e.g., aluminium, carbon, and copper) can be used to manufacture the electrodes. Thus, low-cost and disposable MCE-C4D can be produced and used for fast biochemical assays, diagnostics, homeland security, food safety, and many other applications. On the other hand, the higher limits of detection generally attained with some other detection methods, and the strong influence of the temperature on the C4D signal can be pointed out as the major drawbacks.The wide range of successful applications described in the literature for MCE-C4D demonstrates the versatility of this detection method. Additionally, the fact that commercial detectors are now available suggests that there will be many more applications in the coming years.
Acknowledgements
The authors gratefully thank financial support, research fellowships and also scholarships from CNPq and FAPESP (Grants 2010/08559-9, 2008/57805-2, and 2008/53868-0).References
- P. S. Dittrich, K. Tachikawa and A. Manz, Anal. Chem., 2006, 78, 3887 CrossRef CAS.
- W. K. T. Coltro, E. Piccin, E. Carrilho, D. P. de Jesus, J. A. F. da Silva, H. D. T. da Silva and C. L. do Lago, Quim. Nova, 2007, 30, 1986 CrossRef CAS.
- J. West, M. Becker, S. Tombrink and A. Manz, Anal. Chem., 2008, 80, 4403 CrossRef CAS.
- A. Arora, G. Simone, G. B. Salieb-Beugelaar, J. T. Kim and A. Manz, Anal. Chem., 2010, 82, 4830 CrossRef CAS.
- F. Sassa, K. Morimoto, W. Satoh and H. Suzuki, Electrophoresis, 2008, 29, 1787 CrossRef CAS.
- A. Manz, N. Graber and H. M. Widmer, Sens. Actuators, B, 1990, 1, 244 CrossRef.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2010, 82, 3 CrossRef CAS.
- M. E. Johnson and J. P. Landers, Electrophoresis, 2004, 25, 3513 CrossRef CAS.
- I. M. Lazar, J. Grym and F. Foret, Mass Spectrom. Rev., 2006, 25, 572 CrossRef.
- W. R. Vandaveer, S. A. Pasas-Farmer, D. J. Fischer, C. N. Frankenfeld and S. M. Lunte, Electrophoresis, 2004, 25, 3528 CrossRef CAS.
- D. J. Fischer, M. K. Hulvey, A. R. Regel and S. M. Lunte, Electrophoresis, 2009, 30, 3324 CrossRef CAS.
- D. C. Kirkpatrick, C. Antwi and R. S. Martin, Anal. Methods, 2010, 2, 811 RSC.
- M. Pumera, Talanta, 2007, 74, 358–364 CrossRef CAS.
- P. Kubáň and P. C. Hauser, Anal. Chim. Acta, 2008, 607, 15 CrossRef.
- P. Kubáň and P. C. Hauser, Electrophoresis, 2011, 32, 30 CrossRef.
- W. K. T. Coltro, D. P. de Jesus, J. A. F. da Silva, C. L. do Lago and E. Carrilho, Electrophoresis, 2010, 31, 2487 CrossRef CAS.
- H. Zahn, Z. Phys., 1928, 51, 350 CrossRef CAS.
- T. S. Burkhalter, High Frequency Conductometric (Impedimetric) Titrations, in Comprehensive Analytical Chemistry, IIA—Electrical Methods, ed. C. L. Wilson and D. W. Wilson, Elsevier Publishing Co., Amsterdam, 1964, ch. V Search PubMed.
- E. Pungor, F. Pal and K. Toth, Anal. Chem., 1983, 55, 1728 CrossRef CAS.
- J. F. Alder, P. R. Fielden and A. J. Clark, Anal. Chem., 1984, 56, 985 CrossRef CAS.
- F. Pal, E. Pungor and F. Kovats, Anal. Chem., 1988, 60, 2254 CrossRef CAS.
- B. Gas and J. Vacik, Chem. Listy, 1980, 74, 652 CAS.
- B. Gas, M. Demjanenko and J. Vacik, J. Chromatogr., A, 1980, 192, 253 CrossRef CAS.
- J. Vacik, J. Zuska and I. Muselasova, J. Chromatogr., A, 1985, 320, 233 CrossRef CAS.
- A. J. Zemann, E. Schnell, D. Volgger and G. K. Bonn, Anal. Chem., 1998, 70, 563 CrossRef CAS.
- J. A. F. da Silva and C. L. do Lago, Anal. Chem., 1998, 70, 4339 CrossRef.
- A. J. Zemann, Electrophoresis, 2003, 24, 2125 CrossRef CAS.
- J. G. A. Brito-Neto, J. A. F. da Silva, L. Blanes and C. L. do Lago, Electroanalysis, 2005, 17, 1198 CrossRef CAS.
- J. G. A. Brito-Neto, J. A. F. da Silva, L. Blanes and C. L. do Lago, Electroanalysis, 2005, 17, 1207 CrossRef CAS.
- P. Kubáň and P. C. Hauser, Electrophoresis, 2009, 30, 176 CrossRef.
- V. Šolínová and V. Kašička, J. Sep. Sci., 2006, 29, 1743 CrossRef.
- F. M. Matysik, Microchim. Acta, 2008, 160, 1 CrossRef CAS.
- R. M. Guijt, E. Baltussen, G. van Der Steen, J. Frank, H. A. H. Billiet, T. Schalkhammer, F. Laugere, M. J. Vellekoop, A. Berthold, P. M. Sarro and G. W. K. van Dedem, Electrophoresis, 2001, 22, 2537 CrossRef CAS.
- E. X. Vrouwe, R. Luttge and A. van den Berg, Electrophoresis, 2004, 25, 1660 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, New York, 2001 Search PubMed.
- P. Kubáň and P. C. Hauser, Electrophoresis, 2004, 25, 3387 CrossRef.
- A. Berthold, F. Laugere, H. Schellevis, C. R. de Boer, M. Laros, R. M. Guijt, P. M. Sarro and M. J. Vellekoop, Electrophoresis, 2001, 22, 3511 Search PubMed.
- J. Lichtenberg, N. F. Rooij and E. Verpoorte, Electrophoresis, 2002, 23, 3769 CrossRef CAS.
- W. K. T. Coltro, J. A. F. da Silva and E. Carrilho, Electrophoresis, 2008, 29, 2260 CrossRef CAS.
- J. Tanyanyiwa, E. M. Abad-Villar, T. Fernandéz-Abedul, A. Costa-García, W. Hoffmamn, A. E. Guber, D. Herrmann, A. Gerlach, N. Gottschlich and P. C. Hauser, Analyst, 2003, 128, 1019 RSC.
- J. Wang, G. Chen and A. Muck, Talanta, 2009, 78, 207 CrossRef CAS.
- F. Laugere, R. M. Guijt, J. Bastemeijer, G. van der Steen, A. Berthold, E. Baltussen, P. Sarro, G. W. K. van Dedem, M. J. Vellekoop and A. Bossche, Anal. Chem., 2003, 75, 306 CrossRef CAS.
- C. Y. Lee, C. M. Chen, G. L. Chang, C. H. Lin and L. M. Fu, Electrophoresis, 2006, 27, 5043 CrossRef CAS.
- T. Shang, E. Teng, A. T. Woolley, B. A. Mazzeo, S. M. Schultz and A. R. Hawkins, Electrophoresis, 2010, 31, 2596 CrossRef CAS.
- T. P. Segato, W. K. T. Coltro, A. L. J. Almeida, M. H. O. Piazetta, A. L. Gobbi, L. H. Mazo and E. Carrilho, Electrophoresis, 2010, 31, 2526 CrossRef CAS.
- B. Liu, Q. Jin, Y. Zhang, D. Mayer, H. Krause, J. Zhao and A. Offenhäusser, Microchim. Acta, 2011, 172, 193 CrossRef CAS.
- J. Liu, J. Wang, Z. Chen, Y. Yu, X. Yang, X. Zhang, Z. Xu and C. Liu, Lab Chip, 2011, 11, 969–973 RSC.
- W. K. T. Coltro, J. A. F. da Silva and E. Carrilho, Anal. Methods, 2011, 3, 168 RSC.
- R. M. Guijt, J. P. Armstrong, E. Candish, V. Lefleur, W. J. Percey, S. Shabala, P. C. Hauser and M. C. Breadmore, Sens. Actuators, B, 2011, 159, 307 CrossRef CAS.
- M. Pumera, J. Wang, F. Opekar, I. Jelínek, J. Feldman, H. Löwe and S. Hardt, Anal. Chem., 2002, 74, 1968 CrossRef CAS.
- J. Wang and M. Pumera, Anal. Chem., 2002, 74, 5919 CrossRef CAS.
- J. Tanyanyiwa and P. C. Hauser, Anal. Chem., 2002, 74, 6378 CrossRef CAS.
- J. Wang, G. Chen and A. Muck, Jr, Anal. Chem., 2003, 75, 4475 CrossRef CAS.
- J. Wang, G. Chen, A. Muck, Jr and C. E. Collins, Electrophoresis, 2003, 24, 3728 CrossRef CAS.
- C. L. do Lago, H. D. T. da Silva, C. A. Neves, J. G. A. Brito-Neto and J. A. F. da Silva, Anal. Chem., 2003, 75, 3853 CrossRef CAS.
- C. L. do Lago, C. A. Neves, D. P. de Jesus, H. D. T. da Silva, J. G. A. Brito-Neto and J. A. F. da Silva, Electrophoresis, 2004, 25, 3825 CrossRef CAS.
- Y. Ding, C. D. Garcia and K. R. Rogers, Anal. Lett., 2008, 41, 335 CrossRef CAS.
- J. Wang, G. Chen, M. P. Chatrathi, M. Wang, R. Rinehart and A. Muck, Jr, Electroanalysis, 2008, 20, 2416 CrossRef CAS.
- G. Fercher, A. Haller, W. Smetana and M. J. Vellekoop, Anal. Chem., 2010, 82, 3270 CrossRef CAS.
- Z. Chen, Q. Li, O. Li, X. Zhou, Y. Wei and J. Mo, Talanta, 2007, 71, 1944 CrossRef CAS.
- J. Wang, M. Pumera, G. E. Collins and A. Mulchandani, Anal. Chem., 2002, 74, 6121 CrossRef CAS.
- K. A. Mahabadi, I. Rodriguez, C. Y. Lim, D. K. Maurya, P. C. Hauser and N. F. Rooij, Electrophoresis, 2010, 31, 1063 CAS.
- P. Kubáň and P. C. Hauser, Electrophoresis, 2004, 25, 3398 CrossRef.
- P. Kubáň and P. C. Hauser, Lab Chip, 2005, 5, 407 RSC.
- P. Kubáň and P. C. Hauser, Lab Chip, 2008, 8, 1829 RSC.
- B. Liu, Y. Zhang, D. Mayer, H. Krause, Q. Jin, J. Zhao and A. Offenhäusser, Electrophoresis, 2011, 32, 699 CrossRef CAS.
- J. Tanyanyiwa, E. M. Abad-Villar and P. C. Hauser, Electrophoresis, 2004, 25, 903 CrossRef CAS.
- E. M. Abad-Villar, P. Kubáň and P. C. Hauser, Electrophoresis, 2005, 26, 3609 CrossRef CAS.
- J. Tanyanyiwa and P. C. Hauser, Electrophoresis, 2004, 25, 3010 CrossRef CAS.
- X. Y. Gong and P. C. Hauser, Electrophoresis, 2006, 27, 4375 CrossRef CAS.
- P. Tuma, E. Samková, F. Opekar, V. Jurka and K. Stulik, Electrophoresis, 2007, 28, 2174 CrossRef CAS.
- J. Wang, G. Chen, A. Muck, Jr, M. P. Chatrathi, A. Mulchandani and W. Chen, Anal. Chim. Acta, 2004, 505, 183 CrossRef CAS.
- A. Schuchert-Shi, P. Kubáň and P. C. Hauser, Electrophoresis, 2007, 28, 4690 CrossRef CAS.
- A. Schuchert-Shi and P. C. Hauser, Anal. Biochem., 2008, 376, 262 CrossRef CAS.
- M. Vázquez, C. Frankenfeld, W. K. T. Coltro, E. Carrilho, D. Diamond and S. M. Lunte, Analyst, 2010, 135, 96 RSC.
- P. Kubáň and P. C. Hauser, Electrophoresis, 2005, 26, 3169 CrossRef.
- W. S. Law, P. Kubáň, J. H. Zhao, S. F. Y. Li and P. C. Hauser, Electrophoresis, 2005, 26, 4648 CrossRef CAS.
- H. Mühlberger, W. Hwang, A. E. Guber, V. Saile and W. Hoffmann, IEEE Sens. J., 2008, 8, 572 CrossRef.
- Q. Lu, P. Wu and G. E. Collins, Electrophoresis, 2007, 28, 3485 CrossRef CAS.
- J. J. P. Mark, A. Kumar, H. Demattio, W. Hoffmann, A. Malik and F. M. Matysik, Electroanalysis, 2011, 23, 161–168 CrossRef CAS.
- J. Wang, M. Pumera, G. Collins, F. Opekar and I. Jelínek, Analyst, 2002, 127, 719 RSC.
- Y. Ding and K. R. Rogers, Electroanalysis, 2008, 20, 2192 CrossRef CAS.
- Y. Chen, P. Yang, J. Li, D. Chen and G. Chen, Anal. Bioanal. Chem., 2006, 384, 683 CrossRef CAS.
- Z. Walsh, M. Vazquez, F. Benito-Lopez, B. Paull, M. Macka, F. Svec and D. Diamond, Lab Chip, 2010, 10, 1777 RSC.
Footnote |
| † This article is part of a web theme in Analyst and Analytical Methods on Future Electroanalytical Developments, highlighting important developments and novel applications. Also in this theme is work presented at the Eirelec 2011 meeting, dedicated to Professor Malcolm Smyth on the occasion of his 60th birthday. |
| This journal is © The Royal Society of Chemistry 2012 |
