A label-free cytosensor for the enhanced electrochemical detection of cancer cells using polydopamine-coated carbon nanotubes†‡
Ting-Ting
Zheng
,
Rui
Zhang
,
Lanfang
Zou
and
Jun-Jie
Zhu
*
State Key Laboratory of Analytical Chemistry for Life Science, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, P.R. China. E-mail: jjzhu@nju.edu.cn; Fax: +86-25-8359-4976; Tel: +86-25-8359-4976
First published on 6th December 2011
Abstract
An electrochemical, label-free method was developed to detect folate receptor positive tumor cells by specific recognition of a polydopamine-coated carbon nanotubes–folate nanoprobe to cell-surface folate receptors. This strategy offers great promise to extend its application in studying the interaction of ligand and cell-surface receptor.
As we know, the early detection of tumor cells plays an important part in effective cancer treatment. Some methods such as immunohistochemistry, immunofluorescence, flow cytometry, reverse transcription-polymerase chain reaction (RT-PCR), and immunization-magnetic separation have been established for monitoring cell viability and proliferation.1 Among these methods, the electrochemical sensing method has attracted considerable attention because of its remarkable advantages such as high sensitivity, simplicity, rapid response, compatibility with miniaturization technology, and low cost.2 However, the selectivity for some electrochemical biosensors may be limited, and therefore, sensitive and valuable ligands for selective recognition and capture of target cancer cells are desirable.
With unique chemical and physical properties, carbon nanotubes (CNTs) have generated a tremendous amount of research interest in the area of biosensing, imaging and drug delivery.3 Incorporation of CNTs not only produces new functions but also improves their stability and biocompatibility. Some biomolecules, inorganic materials and polymers as modifiers can be attached onto CNT surfaces.4 Recently, polydopamine (PDA), a mimic of the specialized adhesive foot protein Mefp-5 (Mytilus edulis foot protein-5), has been reported to perform well as a binding agent for coating on various substrates.5
Herein, the spontaneous oxidative polymerization of dopamine (DOA) was employed to fabricate a versatile platform, which provided imide to carry out a coupling reaction with carboxyl from folic acid to obtain folic acid-functionalized nanoprobes (CNTs@PDA-FA) (Scheme 1). The as-prepared CNTs@PDA-FA nanoprobes may not only maintain excellent characteristics as normal carbon nanotubes, but also have a high affinity of folic acid with the folate receptor over-expressed tumor cells. Based on above-mentioned advantages, we present a label-free method for a sensitive detection of HeLa and HL-60 cells with the electrochemical impedance technique. To the best of our knowledge, this is the first work on the use of polydopamine-coated carbon nanotubes for the electrochemical detection of cancer cells.
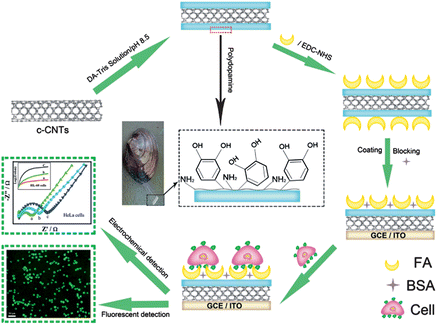 | ||
| Scheme 1 Schematic illustration of the folic acid-targeted cytosensing strategy. | ||
After dopamine was dissolved into pH 8.5 Tris buffer and exposed to air, it turned grey and eventually a polymeric precipitate was observed, polydopamine (PDA), which has an aromatic structure with catechol groups. Fig. 1S(A)‡ shows the reaction formula. In the experiment, this spontaneous oxidative polymerization was employed to fabricate a versatile platform on the CNT surface. Compared the SEM image of the resultant PDA-modified CNTs (CNTs@PDA) and CNTs-COOH (Fig. 2S‡), the surface of CNTs@PDA clearly became rough. A TEM image was also used to characterize the CNT@PDA nanocomposites. As presented in Fig. 1A, all nanotubes are wrapped by polymer shells, which have a lighter color than the graphene sidewall of the CNT due to lower density. The interface of the CNT sidewall and PDA is clearly observed. As shown in Fig. 1B, the shell thickness is about 8.2 nm and gradually increases during reaction.
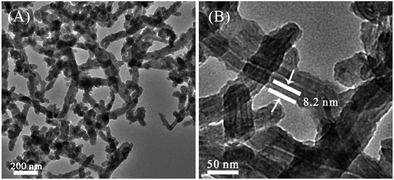 | ||
| Fig. 1 TEM image of CNTs@PDA (A) and high magnification of the CNTs@PDA surface (B), illustrating the uniformity of the coating and the presence of the polydopamine. | ||
Folic acid is a water-soluble vitamin B complex, and its molecular structure is shown in Fig. 1S(B).‡ The CNTs@PDA-FA nanoprobe can be achieved by a coupling reaction between the carbon imide and carboxyl from folic acid. The FTIR spectrum was used to identify the functional groups presented outside the CNTs after the treatment of PDA and FA. The spectrum of CNTs@PDA (Fig. 3S(A)‡ curve b) illustrates that the bands appear at 1606 cm−1 and 1508 cm−1 due to aromatic rings, as well as bands due to methylene –CH2 (2930 cm−1), catechol hydroxyl O–H (3386 cm−1), and aromatic amidocyanogen (1280 cm−1) groups situated outside the CNTs, which indicated that the PDA was coated outside the CNTs. For the CNTs@PDA-FA nanoprobe (curve c), two new peaks at 1225 cm−1 and 1201 cm−1 represent pteridine rings from FA, demonstrating that folic acid has coupled to the CNTs@PDA.
The biocompatibility is related to hydrophilicity, which could be measured by the contact angle technique. As shown in Fig. 3S(B),‡ bare GCE, CNTs-COOH, CNTs@PDA and the CNTs@PDA-FA-modified electrode gave the contact angles of 73.3 ± 1.3°, 45.0 ± 0.2°, 22.9 ± 0.6° and 21.3 ± 1.8°, respectively. The CNTs@PDA-FA gave a smaller contact angle than any other substrate, demonstrating its excellent hydrophilicity. Therefore, the prepared nanoprobe provided a microenvironment highly favorable for cell adhesion and retention of cell activity.
The electrochemical impedance spectra (EIS) of the modified electrodes were shown in Fig. 3S(C).‡ At a bare GCE, the redox process of the probe showed an electron transfer resistance of 400 Ω (curve a). After CNTs@PDA-FA was assembled onto the electrode, the semicircle decreased dramatically (curve b) due to the good conductivity of the CNTs@PDA-FA probe. This result also proved that the conductivity of the carbon nanotubes can be well maintained, and this interface can provide a sensitive response platform for cell electrochemical detection.
Calcein-AM (calcein acetoxymethyl ester), a non-fluorescent, cell-permeable compound, converts to the strongly green fluorescent calcein when hydrolyzed by intracellular esterase in live cells. It is widely used for determining cell viability since the fluorescence intensity of calcein is proportional to the amount of live cells. Calcein-AM staining experiments (Fig. 2) showed that folate receptor positive tumor cells such as HL-60 and HeLa cells were effectively captured by the CNTs@PDA-FA nanoprobe. Most of the capture cells were living (green shows living cells), indicating the capability of the CNT@PDA-FA-modified interface to maintain cells that are live.
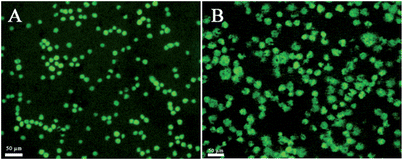 | ||
| Fig. 2 The fluorescent images of the HL-60 (A) and HeLa (B) cells stained by calcein-AM after capture on the ITO/CNTs@PDA-FA/BSA electrode for 1 h. The living cells emit green fluorescence. | ||
Electrochemical impedance spectroscopy (EIS) is a popular method to monitor live cells. The impedance spectra include a semicircle portion and a linear portion. The semicircle portion at higher frequencies corresponds to the electron-transfer-limited process, and the linear portion at lower frequencies represents the diffusion-limited process. The semicircle diameter equals the electron-transfer resistance, Ret. To evaluate the reaction between cells and the interface, the CNTs@PDA-FA-modified electrode was exposed to various concentrations of cells. The corresponding Nyquist plots of impedance spectra are shown in Fig. 3A. The diameter of the Nyquist circle increased with the addition of HeLa cells, implying the significant occupation of more cells on the electrode surface after the cell incubation, leading to larger Ret values for the redox probe due to the decreased active surface area, and the cells' barrier effect against the electron communication between the electrochemical probe and the electrode surface. The Ret value was proportional to the logarithm of HeLa cell concentration ranging from 5.0 × 102 to 5.0 × 106cells mL−1 (Fig. 3B), with a correlation coefficient of 0.99 (n = 3). The limit of detection was calculated to be 5 × 102cells mL−1, which was better than or comparable to that of 8.32 × 103cells mL−1 at an impedance sensor for K562 cells6 and 1.0 × 104cells mL−1 at a piezoelectric immunosensor for Salmonella.7
![(A) Nyquist plots of modified GCE recorded in solutions containing 10 mM [Fe(CN)6]3−/[Fe(CN)6]4− and 1.0 M KCl with the cell concentrations of 5.0 × 102, 5.0 × 103, 5.0 × 104, 5.0 × 105 and 5.0 × 106cells mL−1 (from a to e). (B) Concentration of HeLa cells in the samples versus the electron-transfer resistance (Ret) on the HeLa/BSA/CNTs@PDA-FA/GCE sensors.](/image/article/2012/AN/c2an16023d/c2an16023d-f3.gif) | ||
| Fig. 3 (A) Nyquist plots of modified GCE recorded in solutions containing 10 mM [Fe(CN)6]3−/[Fe(CN)6]4− and 1.0 M KCl with the cell concentrations of 5.0 × 102, 5.0 × 103, 5.0 × 104, 5.0 × 105 and 5.0 × 106cells mL−1 (from a to e). (B) Concentration of HeLa cells in the samples versus the electron-transfer resistance (Ret) on the HeLa/BSA/CNTs@PDA-FA/GCE sensors. | ||
EIS experiments were further performed for monitoring the dynamic recognition process between suspension cells and the electrode interface. In our system, the ac impedance was measured at a potential of 5 mV. Under this condition, there is no oxidation–reduction chemistry taking place and the measurements are a very gentle way of measuring the intrinsic electrical properties of the interface. The charge-transfer process is negligible, and the diffusion-controlled process is an important one. Therefore, the following ac impedance measurements could be used to study the changes of the modified electrode interface impedance at a fixed low frequency (10 Hz). The relative resistance ΔR was defined by ΔR = Rt − R0, where Rt and R0 represent the impedance measured at a fixed time, and when the electrode just was immersed into the solution, respectively. ΔR showed the increasing trend with the increase of time and the increasing rate is related to concentration of HL-60 cells. This trend reflects the slow recognition process between the nanoprobes and HL-60 cells in the solution. The number of HL-60 cells loaded on the electrode interface increased with time, leading to capacitance reduction and impedance enhancement. As shown in Fig. 4A, the impedance increased gradually with time and reached a maximum value at 50 min. Thus, the recognition time between the sensors and cells could be optimized by the impedance–time spectra. The time-dependence of impedance changes is presented in Fig. 4B in terms of the the rate of change of the relative resistance ΔA. ΔA was calculated by ΔA = (ΔRcell − ΔR0)/ΔR0, where ΔRcell and ΔR0 represent the impedance of the modified electrode immersed into the cell solution with different concentrations in PBS buffer for 50 min. The ΔA value was proportional to the logarithm of the HL-60 cell concentration ranging from 5.0 × 103 to 5.0 × 105cells mL−1, with a correlation coefficient of 0.99 (n = 3). The limit of detection was calculated to be 5 × 102cells mL−1, which was better than or comparable to 5 × 103cells mL−1 at a photoelectrochemical cytosensor for SMMC-7721 cells.8
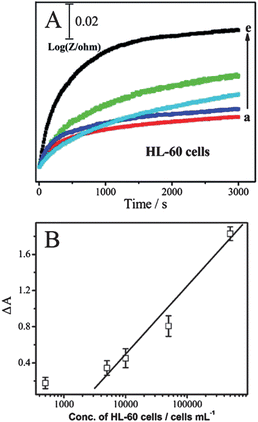 | ||
| Fig. 4 (A) Relative impedance at 10 Hz with time for BSA/CNTs@PDA-FA/GCE scanned while being immersed in various concentrations of HL-60 cells in PBS: (a) 0, (b) 5.0 × 102, (c) 5.0 × 103, (d) 5.0 × 104, (e) 5.0 × 105cells mL−1. (B) Calibration plots of the increase rate of relative impedance (ΔA) for determination HL-60 cells at the BSA/CNTs@PDA-FA/GCE. | ||
Conclusions
This work describes the preparation of a novel CNTs@PDA-FA nanoprobe and the fabrication of a sensitive impedance sensor for the detection of tumor cells, which can detect as low as 500 cells. Compared to other systems that require antibodies or natural enzymes, this composite is more robust and not susceptible to denaturation or decomposition. The as-prepared CNTs@PDA-FA-modified electrode may not only maintain the advantages of the CNTs@PDA nanocomposites, such as conductivity, stability, biocompatibility and reactivity, but also has the high affinity of folic acid with the folate receptor over-expressed tumor cells. The research will facilitate the application of functionalized carbon nanotubes for highly sensitive and selective cell detection, DNA and protein analysis and the development of new imaging modalities.Acknowledgements
We are grateful to the support of ‘973’ Program (No. 2011CB933502), the International S&T Cooperation Projects of China (2010DFA42060), and the Fundamental Research Funds for the Central Universities. We thank the help from Dr Jingjing Zhang and Mr Youkui Huang, Nanjing University.Notes and references
- (a) S. K. Singh, C. Hawkins, I. D. Clarke, J. A. Squire, J. Bayani, T. Hide, R. M. Henkelman, M. D. Cusimano and P. B. Dirks, Nature, 2004, 432, 396–401 CrossRef CAS; (b) D. Schamhart, J. Swinnen, K. H. Kurth, A. Westerhof, R. Kuster, H. Borchers and C. Sternberg, Clin. Chem., 2003, 49, 1458–1466 CrossRef CAS; (c) J. A. Phillips, Y. Xu, Z. Xia, Z. H. Fan and W. H. Tan, Anal. Chem., 2009, 81, 1033–1039 CrossRef CAS.
- Q. Shen, S. K. You, S. G. Park, D. D. Guo, B. A. Chen and X. M. Wang, Electroanal., 2008, 20, 2526–2530 CrossRef CAS.
- (a) A. Safavi, N. Maleki and F. Tajabadi, Analyst, 2007, 132, 54–58 RSC; (b) J. Wang, C. Timchalk and Y. H. Lin, Environ. Sci. Technol., 2008, 42, 2688–2693 CrossRef CAS; (c) L. Meng, J. Jin, G. X. Yang, T. H. Lu, H. Zhang and C. Cai, Anal. Chem., 2009, 81, 7271–7280 CrossRef CAS.
- (a) G. Wei, F. G. Xu, Z. Li and K. D. Jandt, J. Phys. Chem. C., 2011, 115, 11453–11460 CAS; (b) S. Yesil and G. Bayram, Polym. Eng. Sci., 2011, 51, 1286–1300 CrossRef CAS.
- (a) H. Y. Hu, B. Yu, Q. Ye, Y. S. Gu and F. Zhou, Carbon, 2010, 48, 2347–2353 CrossRef CAS; (b) C. H. Tao, S. R. Yang, J. Y. Zhang and J. Q. Wang, Appl. Surf. Sci., 2009, 256, 294–297 CrossRef CAS; (c) H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS.
- L. Ding, D. Du, J. Wu and H. X. Ju, Electrochem. Commun., 2007, 9, 953–958 CrossRef CAS.
- Y. Y. Wong, S. P. Ng, M. H. Ng, S. H. Si, S. Z. Yao and Y. S. Fung, Biosens. Bioelectron., 2002, 17, 676–684 CrossRef CAS.
- Z. Qian, H. J. Bai, G. L. Wang, J. J. Xu and H. Y. Chen, Biosens. Bioelectron., 2010, 25, 2045–2050 CrossRef CAS.
Footnotes |
| † This article is part of a web theme in Analyst and Analytical Methods on Future Electroanalytical Developments, highlighting important developments and novel applications. Also in this theme is work presented at the Eirelec 2011 meeting, dedicated to Professor Malcolm Smyth on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: experimental details and characterization of CNT@PDA-FA nanoprobe. See DOI: 10.1039/c2an16023d |
| This journal is © The Royal Society of Chemistry 2012 |
