Plasmonic nano-necklace arrays via reconstruction of diblock copolymer inverse micelle nanotemplates†
Ji Yong
Lee
a,
Jieun
Lee
a,
Yu Jin
Jang
a,
Juyon
Lee
a,
Yoon Hee
Jang
a,
Saji Thomas
Kochuveedu
a,
Sang Soo
Lee
b and
Dong Ha
Kim
*a
aDepartment of Chemistry and Nano Science, Division of Molecular and Life Sciences, College of Natural Sciences, Ewha Womans University, 11-1 Daehyun-Dong, Seodaemun-Gu, Seoul, 120-750, Korea
bPolymer Hybrid Research Center, Korea Institute of Science and Technology, P.O. Box 131, Cheongryang, Seoul, 130-650, Korea. E-mail: dhkim@ewha.ac.kr; Fax: +82-2-3277-3419; Tel: +82-2-3277-4517
First published on 26th October 2010
Abstract
We present a facile strategy for the generation of plasmonic nano-necklace arrays by introducing metallic precursors into strings of reconstructed inverse micelle nanotemplates which are induced by applying sequential selective solvent treatment and solvent vapour annealing for quasi-hexagonal arrays of poly(styrene-block-2-vinyl pyridine) inverse micelles.
Plasmonic nanostructures have attracted a great deal of interest due to their unique localized surface plasmon resonance (LSPR) properties depending on typical parameters including the size, shape, surrounding environment, and interdistance.1–5 In particular, metallic nanowires are expected to play a critical role as promising candidates in biosensing owing to their enhanced sensitivity and selectivity.6,7
Numerous methods have been suggested to fabricate linear metallic nanostructures such as spontaneous growth, electrospinning, photolithography, and template-based synthesis.8–13 Among template-assisted fabrications, self-assembled block copolymers (BCPs) for utilization as templates have been increasingly exploited due to their capability to induce periodic nanoscale morphology along with the ease and simplicity with which they can be handled.
In recent years, a few seminal works were reported regarding BCP-templated generation of linear inorganic nanostructures.14–16 For instance, Buriak's group has demonstrated an approach to the application of aligned BCPs for the synthesis of metallic nanowiresvia thermal annealing.14,15 Oriented linear patterns of gold (Au), palladium, and platinum with controlled spacing, width, height and composition were synthesized. However, metal line patterns using cationic salts like silver nitrate (AgNO3) were not obtained because of electrostatic repulsion with protonated poly(vinyl pyridine) units. Russell and co-workers prepared continuous metallic nanostructures based on thin films of BCPs by applying an electrochemical etching or direct metal evaporation.16
In this study, we suggest a generalized strategy to induce strings of noble metal nanoparticle (NP) patterns, denoted by nano-necklace arrays, by using either cationic or anionic metal precursors viasolvent annealing and reconstruction of BCP inverse micelle monolayers without the need of any complicated procedure such as thermal annealing, electrochemistry and evaporation. We also apply this methodology to make bimetallic line patterns consisting of Ag and Au, and their LSPR properties are systematically discussed.
The fabrication route to two dimensional arrays of plasmonic nano-necklaces is schematically depicted in Scheme 1. First, quasi-hexagonally packed arrays of polystyrene-block-poly(2-vinyl pyridine) diblock copolymer (PS-b-P2VP; Mn (PS) = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Mn (P2VP) = 16
000 g mol−1, Mn (P2VP) = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, Mw/Mn = 1.09) inverse micelles were prepared from spin coating a 0.5 wt% solution in THF at 2000 rpm onto n-type (100) Si wafers. THF acts as a common solvent for both the PS and P2VP blocks with a relatively high affinity toward PS blocks, leading to inverse micelles in solution. The spin-coated films were exposed to THF solvent vapours at room temperature for ∼20 min in a closed vessel in order to transform the initial configuration into arrays of inverse micelle nanowires. The rearranged nanowire arrays are then immersed in a selective solvent for the core P2VP block, ethanol, for 20 min by which reconstructed toroidal micelles with pores at the center and excrescent P2VP chains at the rims. Such reconstructed micelle arrays can readily accommodate metallic precursors via coordination interaction with nitrogen atoms at P2VP. The samples were immersed in AgNO3 or HAuCl4 ethanol solutions in a sequential or simultaneous manner. Before use, AgNO3 and HAuCl4 ethanol solutions were aged at room temperature in air at least for 24 h. Generally aging is necessary to achieve stability and good dispersion of the metal precursors in the solution. The metal precursors were reduced by UV irradiation, oxygen plasma treatment, or dipping in a reductant (sodium borohydride, NaBH4) solution. The final nanostructures of the strings of NP arrays are designed in two configurations, i.e., (i) neat Ag or Au NP necklace arrays and (ii) composite Ag–Au ones.
500 g mol−1, Mw/Mn = 1.09) inverse micelles were prepared from spin coating a 0.5 wt% solution in THF at 2000 rpm onto n-type (100) Si wafers. THF acts as a common solvent for both the PS and P2VP blocks with a relatively high affinity toward PS blocks, leading to inverse micelles in solution. The spin-coated films were exposed to THF solvent vapours at room temperature for ∼20 min in a closed vessel in order to transform the initial configuration into arrays of inverse micelle nanowires. The rearranged nanowire arrays are then immersed in a selective solvent for the core P2VP block, ethanol, for 20 min by which reconstructed toroidal micelles with pores at the center and excrescent P2VP chains at the rims. Such reconstructed micelle arrays can readily accommodate metallic precursors via coordination interaction with nitrogen atoms at P2VP. The samples were immersed in AgNO3 or HAuCl4 ethanol solutions in a sequential or simultaneous manner. Before use, AgNO3 and HAuCl4 ethanol solutions were aged at room temperature in air at least for 24 h. Generally aging is necessary to achieve stability and good dispersion of the metal precursors in the solution. The metal precursors were reduced by UV irradiation, oxygen plasma treatment, or dipping in a reductant (sodium borohydride, NaBH4) solution. The final nanostructures of the strings of NP arrays are designed in two configurations, i.e., (i) neat Ag or Au NP necklace arrays and (ii) composite Ag–Au ones.
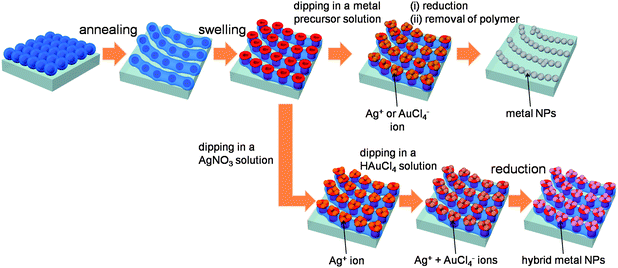 | ||
| Scheme 1 Schematic diagram of the fabrication route to generate plasmonic nano-necklace arrays utilizing BCP inverse micelle thin films as templates and by applying stepwise solvent annealing and surface reconstruction. The arrays of BCP inverse micelles are prepared from spin coating a THF solution on solid substrates. The spin-coated films are exposed to THF solvent vapours at room temperature in a closed vessel in order to transform the nanodot arrays into rearranged nanowire arrays. The arrays are then immersed in a selective solvent for the core P2VP blocks, by which strings of reconstructed toroidal micelles with pores at the center and excrescent P2VP chains at the rims are obtained. Two different strategies are employed to obtain plasmonic nano-necklace arrays in different configurations. Pure metallic nano-necklace arrays consisting of either Ag or Au are generated after immersion into a metal precursor solution, followed by simultaneous reduction of the precursor and removal of polymer templates. On the other hand, hybrid plasmonic nano-necklace arrays consisting of Ag and Au surrounded with polymer matrix are obtained by immersing the films in two metal precursor solutions in a sequential order, followed by reduction of the precursors. | ||
We first discuss about the structural evolution of plasmonic nano-necklace arrays composed of a single component noble metals. Fig. 1a shows a height contrast AFM image of the surface of a PS-b-P2VP inverse micelle monolayer film after spin-coating. The film thickness is about 40 nm as measured by a sectional analysis using AFM software. Quasi-hexagonally packed arrays of inverse micelles are generated. The average center-to-center distance between the neighboring nanodots was measured to be ∼35 nm as calculated from the power spectral density profile (see Fig. S1 in the ESI†). The surface morphology after solvent annealing treatment clearly shows that the nanodot arrays are transformed into nanowire arrays driven by the extra mobility imparted by the THF vapors, as shown in Fig. 1b. As a result, the two-dimensional nanowire arrays have hills and valleys which are arranged with a diameter of about 25 nm and period of 45 nm. When these films are immersed into ethanol, a selective solvent for the P2VP, reconstruction of the film occurs. The hydrophilic blocks located inside of the nanowires are exposed to the external surface and nanowire arrays having nanopores regularly spaced and aligned along the hills are obtained with the lateral spacing reserved, the same as shown in Fig. 1c. From this result, it is revealed that the nanowires in Fig. 1b are composed strings of inverse micelles, which turn into discrete inverted micelle necklaces by the subsequent selective solvent treatment. Immersion in an acidic solution such as HCl or HF solution can also lead to the inversion of inverse micelles during which the nitrogen atoms in pyridine units are protonated. These films, however, cannot accommodate cationic metallic salts, AgNO3, due to electrostatic repulsion. On the other hand, immersion in ethanol leads to P2VP reconstruction by keeping its charge state as neutral, thus rendering the P2VP units the ability to bind with any metallic salts. Ag nano-necklace arrays were obtained by immersing the film in Fig. 1c in a 1 wt% AgNO3 ethanol solution for 1 h (Fig. 1d) followed by exposing it to a UV light with a wavelength of 254 nm for 3 h, resulting in simultaneous reduction of the precursor and removal of polymer templates as shown in Fig. 1e. The chemical identity of Ag NPs was confirmed by the appearance of the localized surface plasmon resonance (SPR) band at about 430 nm from the UV-visible absorption spectrum (Fig. 1f). As another example of a single component nanostructure, Au nano-necklace arrays were also prepared following the same procedure except for the use of HAuCl4 as precursors. The nanoring arrays in Fig. 1c were immersed into a 1 wt% HAuCl4 ethanol solution for 1 h (Fig. 1g) followed by an oxygen plasma treatment for 1 min (Fig. 1h), yielding the final Au nano-necklace arrays. The characteristic LSPR band of Au nanostructures is also observed at about 530 nm from the UV-visible absorption spectrum (Fig. 1i). The AFM images of the final plasmonic nanostructures in Fig. 1e and h clearly reveal that strings of discrete metal NPs have been generated.
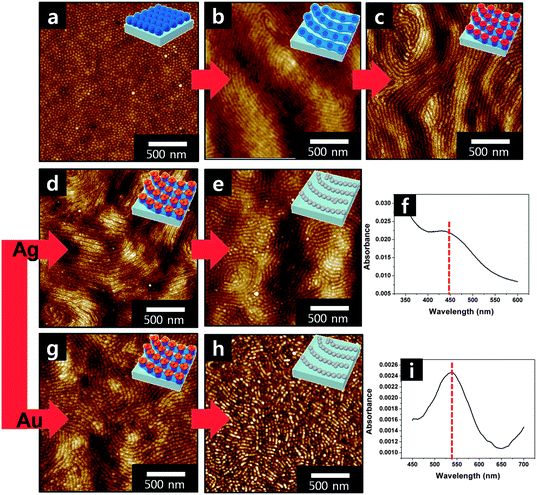 | ||
| Fig. 1 (a) Height contrast AFM image of the surface of a PS-b-P2VP inverse micelle monolayer film after spin-coating; (b) nanowire arrays after solvent annealing treatment; (c) strings of reconstructed toroidal micelle arrays having nanopores obtained by immersion into ethanol; (d) reconstructed micelle nanoring arrays after immersion into a AgNO3 ethanol solution; (e) Ag nano-necklace arrays obtained by exposing the films to a UV light; (f) UV-visible absorption spectrum obtained from the Ag nano-necklace arrays; (g) the reconstructed micelle nanoring arrays after immersion into a HAuCl4 ethanol solution; (h) Au nano-necklace arrays obtained by oxygen plasma treatment; and (i) UV-visible absorption spectrum obtained from the Au nano-necklace arrays. | ||
We also demonstrate that multi-component metallic nanostructures can be induced by dipping reconstructed inverse micelle nanotemplates in Fig. 1c into metal precursor solutions in a sequential manner. The AFM image of reconstructed micelle nanoring arrays after immersion into a AgNO3 ethanol solution is displayed in Fig. 2a and the one subsequently immersed into a HAuCl4 ethanol solution is shown in Fig. 2b. It was found that the sequence of immersion in two metal precursor solutions does not make any differences. For the film containing two metallic precursors, the reduction is accomplished by treating it with a 0.01 M NaBH4 ethanol solution. Thus, hybrid plasmonic nano-necklace arrays consisting of Ag and Au surrounded with polymer matrix were obtained as shown in Fig. 2c.
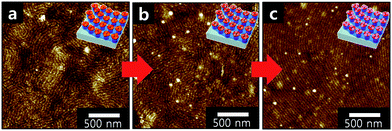 | ||
| Fig. 2 (a) Height contrast AFM image of strings of reconstructed micelle nanoring arrays after immersion into a AgNO3 ethanol solution; (b) reconstructed micelle nanoring arrays after subsequently immersed into a HAuCl4 ethanol solution; and (c) hybrid plasmonic nano-necklace arrays consisting of Ag and Au surrounded with polymer matrix obtained by reducing the precursors in a NaBH4 ethanol solution. | ||
The chemical composition of the final nanostructure was investigated by X-ray photoelectron spectroscopy (Fig. 3). The peaks at 373.7 eV and 367.7 eV in Fig. 3a are assigned to Ag 3d3/2 and Ag 3d5/2, respectively. In Fig. 3b, characteristic peaks of Au are also observed at 87.2 eV and 83.6 eV, which are ascribed to Au 4f5/2 and Au 4f7/2, respectively. The XPS results indicate that the resultant film has both Ag and Au moieties.
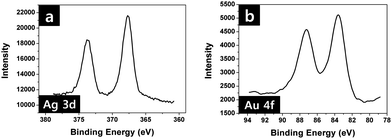 | ||
| Fig. 3 High resolution XPS spectra of (a) Ag3d and (b) Au4f obtained from the hybrid plasmonic nano-necklace arrays consisting of Ag and Au surrounded with polymer matrix in Fig. 2c. | ||
UV-visible absorption spectrum obtained from the hybrid film containing both Ag and Au exhibits one characteristic LSPR band centered at ∼474 nm instead of two separate peaks as shown in Fig. 4. If we recall that Ag- or Au-containing nanostructure has its distinct bands at 430 nm (Fig. 1f) and 530 nm (Fig. 1i), respectively, it is concluded that the hybrid nanostructure fabricated in this study (Fig. 2c) is a typical alloy of Ag and Au considering that all the Ag–Au alloys exhibit one peak located between the ones for pure Ag and Au nano-objects.
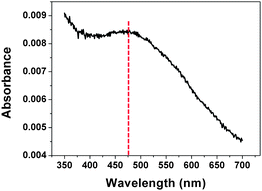 | ||
| Fig. 4 UV-visible absorption spectrum obtained from the hybrid plasmonic nano-necklace arrays consisting of Ag and Au surrounded with polymer matrix. | ||
In summary, we suggested a versatile strategy to fabricate highly dense, two dimensional plasmonic nano-necklace arrays utilizing BCP inverse micelle thin films as templates and by applying solvent annealing and surface reconstruction. The resulting nanostructures were designed in two different configurations, i.e., pure metallic nano-necklace arrays or strings of metallic NPs surrounded with polymer matrix. Thus, viable experimental protocols to construct plasmonic nanostructures with unique architecture, tailored composition and controlled dielectric environments are established. Such exquisite metal nanostructures may find great promise for functional plasmonic, photonic and catalytic applications.
Acknowledgements
This work was supported by National Research Foundation (NRF) of Korea Grant funded by the Korean Government (Nos. 20100000638, 20100001480, 2010-00134) and by the Converging Research Center Program through the Ministry of Education, Science and Technology (2010K000990).References
- Y. Sun and Y. Xia, Analyst, 2003, 128, 686 RSC.
- Y. Xiang, X. Wu, D. Liu, L. Feng, K. Zhang, W. Chu, W. Zhou and S. Xie, J. Phys. Chem. C, 2008, 112, 3203 CrossRef CAS.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857 CrossRef CAS.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef CAS.
- C. Noguez, J. Phys. Chem. C, 2007, 111, 3806 CrossRef CAS.
- (a) K. M. Byun, S. J. Yoon, D. Kim and S. J. Kim, Opt. Lett., 2007, 32, 1902 Search PubMed; (b) K. M. Byun, S. J. Yoon, D. Kim and S. J. Kim, J. Opt. Soc. Am. A, 2007, 24, 522 CrossRef CAS.
- H. J. Lee, A. W. Wark and R. M. Corn, Analyst, 2008, 133, 596 RSC.
- X. Y. Kong and Z. L. Wang, Nano Lett., 2003, 3, 1625 CrossRef CAS.
- R. C. Furneaux, W. R. Rigby and A. P. Davidson, Nature, 1989, 337, 147 CrossRef CAS.
- A. Frenot and L. S. Chronakis, Curr. Opin. Colloid Interface Sci., 2003, 8, 64 CrossRef CAS.
- Y. Xia, J. A. Rogers, K. E. Paul and G. M. Whitesides, Chem. Rev., 1999, 99, 1823 CrossRef CAS.
- Y. S. Jung, J. H. Lee, J. Y. Lee and C. A. Ross, Nano Lett., 2010, 10, 3722 CrossRef CAS.
- T. T. Albrecht, J. Schotter, G. A. Kastle, N. Emley, T. Shibauchi, L. K. Elbaum, K. Guarini, C. T. Black, M. T. Tuominen and T. P. Russell, Science, 2000, 290, 2126 CrossRef CAS.
- J. Chai and J. M. Buriak, ACS Nano, 2008, 2, 489 CrossRef CAS.
- J. Chai, D. Wang, X. Fan and J. M. Buriak, Nat. Nanotechnol., 2007, 2, 500 CrossRef.
- S. Park, B. Kim, A. Cirpan and T. P. Russell, Small, 2009, 5, 1343 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The power spectral density profile calculated from the AFM images in Fig. 1a. See DOI: 10.1039/c0sm00972e |
| This journal is © The Royal Society of Chemistry 2011 |
