Divergent reactions of indoles with aminobenzaldehydes: indole ring-opening vs. annulation and facile synthesis of neocryptolepine†
Matthew K.
Vecchione
,
Aaron X.
Sun
and
Daniel
Seidel
*
Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, Piscataway, New Jersey 08854, USA. E-mail: seidel@rutchem.rutgers.edu
First published on 19th August 2011
Abstract
Indoles display a diverse pattern of reactivity upon reaction with different classes of aminobenzaldehydes. Whereas the acid-promoted reaction with secondary aminobenzaldehyde leads to indole annulation followed by spontaneous oxidation to neocryptolepine and analogues, the reaction with primary aminobenzaldehydes results in the formation of synthetically useful quinolinesvia a remarkably facile indole ring-opening.
The indole ring system is a privileged scaffold in medicinal chemistry1 and is found in numerous natural products.2 A particularly attractive way of generating new indole-containing molecular frameworks is the annulation of preformed and readily available indoles. The most common annulation of indoles involves the formation of carbazoles,3 and various indole annulations that lead to dearomatization of the indole nucleus have been disclosed.4 Other annulations with (partially) saturated rings in which indole retains its aromaticity have also been reported.5 Here we introduce a new acid-catalyzed indole annulation with secondary aminobenzaldehydes that allows for the rapid synthesis of neocryptolepine and various analogues. In addition, we describe an unprecedented reaction of primary aminobenzaldehydes with indoles that results in the formation of quinolinesvia a remarkably facile indole ring-opening process.
As part of a program aimed at developing redox-neutral reaction cascades for the rapid buildup of molecular complexity,6 we recently reported a new one-step indole annulation to form indole-fused benzazepines such as 2.6h As outlined in Fig. 1, the diphenyl phosphate (DPP) catalyzed reaction of tertiary aminobenzaldehyde 1 with indole initially results in the formation of the vinylogous iminium (azafulvenium) ion 3. This species then undergoes an intramolecular 1,5-hydride transfer to form iminium ion 4. Subsequent ring-closure and proton loss results in the formation of indolobenzazepine 2.
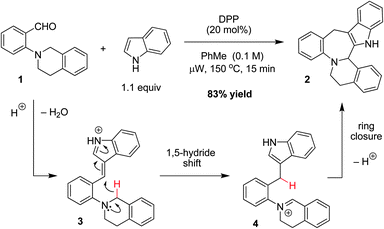 | ||
| Fig. 1 Redox-neutral indole annulation. | ||
In considering alternative reaction scenarios, it occurred to us that replacement of the tertiary aminobenzaldehyde (e.g., 1) for a secondary aminobenzaldehyde might dramatically alter the course of the reaction. As a particularly intriguing possibility, we proposed the one-step conversion of N-methyl aminobenzaldehyde (5a)7 to the natural product neocryptolepine (6a, also referred to as cryptotackieine).8 As outlined in Fig. 2 and in analogy to the process depicted in Fig. 1, condensation of 5a with indole is expected to form azafulvenium ion 7. Rather than engaging in an intramolecular hydride transfer event, 7 is envisioned to undergo direct ring-closure to form 8 or its corresponding indole tautomer (not shown). Subsequent oxidation of 8 completes the synthesis of neocryptolepine (6a), a bioactive material that has been identified as a promising lead for new antimalarial agents.9 Not surprisingly, the development of efficient methods that provide access to analogues of neocryptolepine remains of significant interest.10
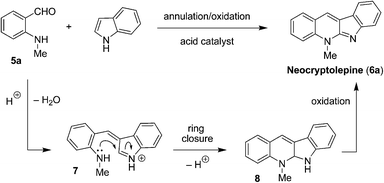 | ||
| Fig. 2 Proposed synthesis of neocryptolepine (6a). | ||
We initiated our studies by evaluating the reaction of N-methyl aminobenzaldehyde (5a) with indole under a variety of conditions. Gratifyingly, an efficient procedure for the formation of neocryptolepine (6a) was rapidly identified (Fig. 3). While a number of acids such as DPP and trifluoroacetic acid (TFA) were viable promoters, para-toluenesulfonic acid (p-TSA) gave rise to the best results. Accordingly, the condensation of 5a with indole in the presence of p-TSA in ethanol under reflux provided neocryptolepine (6a) in 77% yield. One equivalent of p-TSA was required to achieve full conversion of N-methyl aminobenzaldehyde (5a). Interestingly, the oxidized product 6a was obtained directly. The putative intermediate 8 was not isolated, presumably because it undergoes facile oxidation by air. This approach was amenable to the one-step synthesis of various neocryptolepine analogues (Fig. 3). Electronically diverse indoles readily engaged in the annulation/oxidation cascade to provide compounds 6 in moderate to good yields. Modified aminobenzaldehydes were also viable substrates for this transformation.
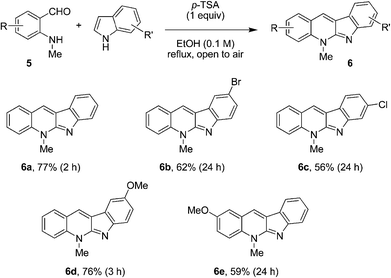 | ||
| Fig. 3 Synthesis of neocryptolepine and analogues. | ||
Next, we sought to expand the substrate scope to primary aminobenzaldehydes. Surprisingly, reactions of unsubstituted aminobenzaldehyde 9a with indole, conducted under a variety of conditions, did not lead to the formation of the expected neocryptolepine analogue 10a or its known tautomer 10b (eqn (1)).11 Rather, we observed the formation of the unexpected compound 11a, the product of an apparent indole ring-opening. Upon heating 9a with indole under reflux in toluene in the presence of one equivalent of p-TSA, 11a was obtained in 83% yield.
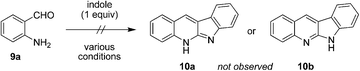 | (1) |
The proposed mechanism for the formation of 11a is shown in Fig. 4. In analogy to tertiary and secondary aminobenzaldehydes, the condensation of indole and 9a initially results in the formation of azafulvenium ion 12. Subsequent ring closure provides intermediate 13, the equivalent of compound 8 in the reaction of indole with N-methyl aminobenzaldehyde (Fig. 2). At this point, the reaction pathway diverges. Rather than undergoing proton loss followed by oxidation to give putative product 10, intra- or intermolecular proton transfer gives rise to species 14. This intermediate then aromatizes to form quinoline 11a upon opening of the indole ring.12 Thus, this reaction can be considered a variant of the Friedländer condensation13,14 in which indole serves as an equivalent of the elusive 2-(2-aminophenyl)acetaldehyde. Whereas a few reports on the ring opening of 3-ketoindoles have appeared,15 the nonoxidative ring-opening of simple indole has essentially been limited to the well-known indole oligomerizations.16,17
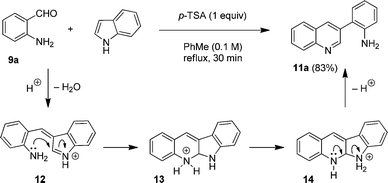 | ||
| Fig. 4 Proposed mechanism for the indole ring-opening. | ||
Intrigued by the facile nature of the quinoline formation, we set out to explore the scope of this indole-opening reaction. As summarized in Fig. 5, electronically diverse indoles participated in reactions with aminobenzaldehyde 9a to form 3-(2-aminophenyl)quinolines 11a–h in good to excellent yields and typically brief reaction times. Substitution of various positions of the indole nucleus was well tolerated. Remarkably, even 2-phenyl indole engaged in this reaction to give product 11h in 64% yield. A prolonged reaction time of 8 h was required for this sterically encumbered substrate. Depending on the nature of the substrate, either p-TSA or TFA proved to be the best acid promoter.
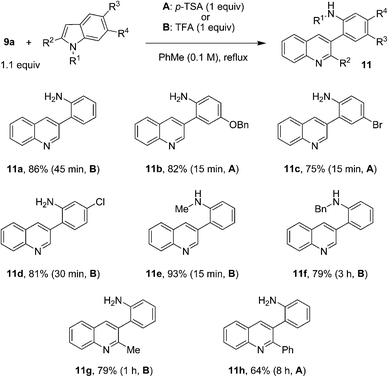 | ||
| Fig. 5 Reactions of aminobenzaldehyde with various indoles. | ||
In order to further expand the substrate scope of the quinoline formation, a number of primary aminobenzaldehydes were allowed to react with indole under the optimized conditions (Fig. 6). Again, various substitution patterns were readily accommodated. The electronic nature of the aminobenzaldehydes appeared to have little impact on the reaction outcome and products were obtained rapidly in generally good to excellent yields.
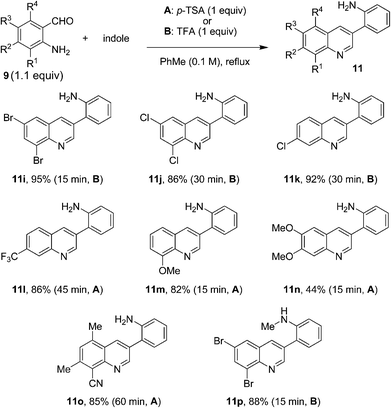 | ||
| Fig. 6 Reactions of indole with various aminobenzaldehydes. | ||
Interestingly, 3-(2-aminophenyl)quinoline 11a had previously been prepared via a multistep sequence and used as a starting material in the synthesis of cryptosanguinolentine (also referred to as isocryptolepine).10b,18 This sequence involved azide formation via a diazonium salt, nitrene formation and C–H insertionviathermolysis, and finally alkylation. We envisioned that 11a might be converted to either cryptosanguinolentine or neocryptolepine in a single operation. As outlined in Fig. 7, regioselective methylation of 11a is expected to form quinolinium salt 15. Subsequent 1,4-addition of the pendant aniline, followed by oxidation would provide cryptosanguinolentine. Alternatively, 1,2-addition/oxidation would result in the formation of neocryptolepine (6a).
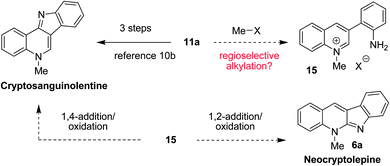 | ||
| Fig. 7 Proposed conversion of 11a to neocryptolepine and cryptosanguinolentine. | ||
To put this hypothesis to the test, 11a was exposed to an excess of methyl iodide under reflux in acetonitrile in a flask kept open to air (eqn (2)). Under these unoptimized conditions, neocryptolepine (6a) was obtained in 64% yield and, at most, traces of cryptosanguinolentine were observed. In preliminary experiments, this strategy was applied to alkylations of 11a using ethyl iodide and benzyl bromide (eqn (3) and (4)). Although the yields for the corresponding N-ethyl and N-benzyl products 16 and 17 remain to be optimized further, this approach appears to be a viable pathway for the synthesis of various neocryptolepine analogues. Furthermore, it is conceivable that alternate reaction conditions can be developed that will allow for the synthesis of cryptosanguinolentine and related products.
 | (2) |
 | (3) |
 | (4) |
In summary, we have uncovered a diverse pattern of reactivity in reactions of indole with aminobenzaldehydes.19 As reported previously, indolobenzazepines form in acid-catalyzed annulation cascades of indole with tertiary aminobenzaldehydes. In contrast, the acid-promoted reaction of indole with secondary aminobenzaldehydes triggers annulation/oxidation cascades that enable the rapid formation of neocryptolepine and analogues. Conversely, under very similar reaction conditions, primary aminobenzaldehydes react with indolesvia a rare indole ring-opening process that results in the formation of synthetically useful 3-(2-aminophenyl)quinolines. The identification of mild reaction conditions for the remarkably facile ring-opening of simple indoles is expected to facilitate the development of other synthetic applications in which indole serves as an equivalent of 2-(2-aminophenyl)acetaldehyde.
Acknowledgements
Financial support from Rutgers, The State University of New Jersey is gratefully acknowledged. We thank Dr Tom Emge for crystallographic analysis and Mr. Michael C. Haibach for helpful discussions. A. X. S. acknowledges support from the Aresty Research Center for Undergraduates. D. S. is a fellow of the A. P. Sloan Foundation and the recipient of an Amgen Young Investigator Award.Notes and references
- For selected reviews, see: (a) F. R. D. Alves, E. J. Barreiro and C. A. M. Fraga, Mini-Rev. Med. Chem., 2009, 9, 782 CrossRef; (b) M. E. Welsch, S. A. Snyder and B. R. Stockwell, Curr. Opin. Chem. Biol., 2010, 14, 347 CrossRef CAS; (c) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS.
- For selected reviews on indole chemistry, see: (a) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045 RSC; (b) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS; (c) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS; (d) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS; (e) A. Palmieri, M. Petrini and R. R. Shaikh, Org. Biomol. Chem., 2010, 8, 1259 RSC.
- (a) L. Lee, J. K. Snyder, in Advances in Cycloaddition, Vol. 6 (ed. M. Harmata), JAI, Stamford, CT, 1999, p. 119 Search PubMed; (b) H. J. Knolker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef.
- For recent examples, see: (a) B. Bajtos, M. Yu, H. D. Zhao and B. L. Pagenkopf, J. Am. Chem. Soc., 2007, 129, 9631 CrossRef CAS; (b) J. Barluenga, E. Tudela, A. Ballesteros and M. Tomas, J. Am. Chem. Soc., 2009, 131, 2096 CrossRef CAS; (c) Y. J. Lian and H. M. L. Davies, J. Am. Chem. Soc., 2010, 132, 440 CrossRef CAS; (d) L. M. Repka, J. Ni and S. E. Reisman, J. Am. Chem. Soc., 2010, 132, 14418 CrossRef CAS; (e) F. J. Robertson, B. D. Kenimer and J. Wu, Tetrahedron, 2011, 67, 4327 CrossRef CAS.
- For examples, see: (a) A. Arcadi, G. Bianchi, M. Chiarini, G. D'Anniballe and F. Marinelli, Synlett, 2004, 944 CrossRef CAS; (b) C. Ferrer, C. H. M. Amijs and A. M. Echavarren, Chem.–Eur. J., 2007, 13, 1358 CrossRef CAS; (c) C. H. M. Amijs, V. Lopez-Carrillo, M. Raducan, P. Perez-Galan, C. Ferrer and A. M. Echavarren, J. Org. Chem., 2008, 73, 7721 CrossRef CAS; (d) A. C. Silvanus, S. J. Heffernan, D. J. Liptrot, G. Kociok-Kohn, B. I. Andrews and D. R. Carbery, Org. Lett., 2009, 11, 1175 CrossRef CAS; (e) Y. H. Lu, X. W. Du, X. H. Jia and Y. H. Liu, Adv. Synth. Catal., 2009, 351, 1517 CrossRef CAS; (f) R. R. Liu and J. L. Zhang, Adv. Synth. Catal., 2011, 353, 36 CrossRef CAS.
- (a) C. Zhang, C. K. De, R. Mal and D. Seidel, J. Am. Chem. Soc., 2008, 130, 416 CrossRef CAS; (b) S. Murarka, C. Zhang, M. D. Konieczynska and D. Seidel, Org. Lett., 2009, 11, 129 CrossRef CAS; (c) C. Zhang, S. Murarka and D. Seidel, J. Org. Chem., 2009, 74, 419 CrossRef CAS; (d) S. Murarka, I. Deb, C. Zhang and D. Seidel, J. Am. Chem. Soc., 2009, 131, 13226 CrossRef CAS; (e) C. Zhang and D. Seidel, J. Am. Chem. Soc., 2010, 132, 1798 CrossRef CAS; (f) I. Deb and D. Seidel, Tetrahedron Lett., 2010, 51, 2945 CrossRef CAS; (g) C. Zhang, D. Das and D. Seidel, Chem. Sci., 2011, 2, 233 RSC; (h) M. C. Haibach, I. Deb, C. K. De and D. Seidel, J. Am. Chem. Soc., 2011, 133, 2100 CrossRef CAS; (i) I. Deb, D. Das and D. Seidel, Org. Lett., 2011, 13, 812 CrossRef CAS; (j) I. Deb, D. J. Coiro and D. Seidel, Chem. Commun., 2011, 47, 6473 RSC.
- I. A. Apple and O. Meth-Cohn, ARKIVOC, 2002, 4 CAS.
- Isolation papers: (a) M. H. M. Sharaf, P. L. Schiff, A. N. Tackie, C. H. Phoebe and G. E. Martin, J. Heterocycl. Chem., 1996, 33, 239 CrossRef CAS; (b) K. Cimanga, T. DeBruyne, L. Pieters, M. Claeys and A. Vlietinck, Tetrahedron Lett., 1996, 37, 1703 CrossRef CAS. For a review on the isolation, biological activities and synthesis of indoloquinoline alkaloids, see: (c) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Curr. Org. Chem., 2011, 15, 1036 CrossRef CAS.
- (a) C. Bailly, W. Laine, B. Baldeyrou, M.-C. De Pauw-Gillet, P. Colson, C. Houssier, K. Cimanga, S. Van Miert, A. J. Vlietinck and L. Pieters, Anti-Cancer Drug Des., 2000, 15, 191 CAS; (b) T. H. M. Jonckers, S. van Miert, K. Cimanga, C. Bailly, P. Colson, M. C. De Pauw-Gillet, H. van den Heuvel, M. Claeys, F. Lemiere, E. L. Esmans, J. Rozenski, L. Quirijnen, L. Maes, R. Dommisse, G. L. F. Lemiere, A. Vlietinck and L. Pieters, J. Med. Chem., 2002, 45, 3497 CrossRef CAS. For a review on indoloquinolines as scaffolds for drug discovery, see: (c) J. Lavrado, R. Moreira and A. Paulo, Curr. Med. Chem., 2010, 17, 2348 CrossRef CAS.
- For previous approaches to neocryptolepine, see: (a) M. Alajarin, P. Molina and A. Vidal, J. Nat. Prod., 1997, 60, 747 CrossRef CAS; (b) G. Timari, T. Soos and G. Hajos, Synlett, 1997, 1067 CrossRef CAS; (c) P. Molina, P. M. Fresneda and S. Delgado, Synthesis, 1999, 326 CrossRef CAS; (d) P. M. Fresneda, P. Molina and S. Delgado, Tetrahedron, 2001, 57, 6197 CrossRef CAS; (e) T. L. Ho and D. G. Jou, Helv. Chim. Acta, 2002, 85, 3823 CrossRef CAS; (f) G. S. M. Sundaram, C. Venkatesh, U. K. S. Kumar, H. Ila and H. Junjappa, J. Org. Chem., 2004, 69, 5760 CrossRef CAS; (g) R. Engqvist and J. Bergman, Org. Prep. Proced. Int., 2004, 36, 386 CrossRef CAS; (h) O. Amiri-Attou, T. Terme and P. Vanelle, Synlett, 2005, 3047 CAS; (i) T. Dhanabal, R. Sangeetha and P. S. Mohan, Tetrahedron, 2006, 62, 6258 CrossRef CAS; (j) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Tetrahedron Lett., 2007, 48, 7870 CrossRef CAS; (k) F. Portela-Cubillo, J. S. Scoto and J. C. Walton, J. Org. Chem., 2008, 73, 5558 CrossRef CAS; (l) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, J. Org. Chem., 2009, 74, 8369 CrossRef CAS; (m) M. J. Haddadin, R. M. B. Zerdan, M. J. Kurth and J. C. Fettinger, Org. Lett., 2010, 12, 5502 CrossRef CAS; (n) G. A. Kraus and H. Guo, Tetrahedron Lett., 2010, 51, 4137 CrossRef CAS; (o) S. Hostyn, K. A. Tehrani, F. Lemiere, V. Smout and B. U. W. Maes, Tetrahedron, 2011, 67, 655 CrossRef CAS.
- S. J. Holt and V. Petrow, J. Chem. Soc., 1948, 922 RSC.
- Fascinatingly, Hoschek investigated the reaction of amino-benzaldehyde 9a with indole almost a century ago: A. B. Hoschek, Ber. Dtsch. Chem. Ges., 1916, 49, 2584 CrossRef CAS . Upon heating 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixtures of 9a and indole neat for 4 h at 140–160 °C, he obtained the corresponding bis-indolylmethane for which he reported a melting point of 97 °C. For comparison, compound 11a melts between 119–120 °C.
2 mixtures of 9a and indole neat for 4 h at 140–160 °C, he obtained the corresponding bis-indolylmethane for which he reported a melting point of 97 °C. For comparison, compound 11a melts between 119–120 °C. - For leading references on the Friedländer reaction, see: (a) P. Friedländer, Ber. Dtsch. Chem. Ges., 1882, 15, 2572 CrossRef; (b) P. Friedländer and C. F. Gohring, Ber. Dtsch. Chem. Ges., 1883, 16, 1833 CrossRef; (c) J. Marco-Contelles, E. Perez-Mayoral, A. Samadi, M. D. Carreiras and E. Soriano, Chem. Rev., 2009, 109, 2652 CrossRef CAS.
- See also: (a) L. Li and D. Seidel, Org. Lett., 2010, 12, 5064 CrossRef CAS; (b) L. Li and D. Seidel, Synthesis, 2011, 1853 CAS , and references cited therein.
- (a) E. E. Garcia, J. G. Riley and R. I. Fryer, J. Org. Chem., 1968, 33, 2868 CrossRef CAS; (b) A. V. Kolotaev, L. I. Belen'kii, A. S. Kononikhin and M. M. Krayushkin, Russ. Chem. Bull., 2006, 55, 892 CrossRef CAS; (c) V. Colotta, D. Catarzi, F. Varano, F. Capelli, O. Lenzi, G. Filacchioni, C. Martini, L. Trincavelli, O. Ciampi, A. M. Pugliese, F. Pedata, A. Schiesaro, E. Morizzo and S. Moro, J. Med. Chem., 2007, 50, 4061 CrossRef CAS; (d) S. Lee and S. B. Park, Org. Lett., 2009, 11, 5214 CrossRef CAS; (e) I. Knepper, V. O. Iaroshenko, M. Vilches-Herrera, L. Domke, S. Mkrtchyan, M. Zahid, A. Villinger and P. Langer, Tetrahedron, 2011, 67, 5293 CrossRef CAS.
- For leading references on indole oligomerization, see: (a) K. Keller, Ber. Dtsch. Chem. Ges., 1913, 46, 726 CrossRef CAS; (b) H. Ishii, K. Murakami, E. Sakurada, K. Hosoya and Y. Murakami, J. Chem. Soc., Perkin Trans. 1, 1988, 2377 RSC; (c) H. Ishii, E. Sakurada, K. Murakami, S. Takase and H. Tanaka, J. Chem. Soc., Perkin Trans. 1, 1988, 2387 RSC.
- For an intriguing synthesis of indolesviapyridine ring-opening, see: A. M. Kearney and C. D. Vanderwal, Angew. Chem., Int. Ed., 2006, 45, 7803 CrossRef CAS.
- For selected approaches to cryptosanguinolentine, see: (a) R. N. Kumar, T. Suresh and P. S. Mohan, Tetrahedron Lett., 2002, 43, 3327 CrossRef CAS; (b) T. H. Jonckers, B. U. W. Maes, G. L. F. Lemiere, G. Rombouts, L. Pieters, A. Haemers and R. A. Dommisse, Synlett, 2003, 615 CAS; (c) T. Dhanabal, R. Sangeetha and P. S. Mohan, Tetrahedron Lett., 2005, 46, 4509 CrossRef CAS; (d) Y. Miki, M. Kuromatsu, H. Miyatake and H. Harnamoto, Tetrahedron Lett., 2007, 48, 9093 CrossRef CAS; (e) C. Meyers, G. Rombouts, K. T. J. Loones, A. Coelho and B. U. W. Maes, Adv. Synth. Catal., 2008, 350, 465 CrossRef CAS; (f) G. A. Kraus, H. Guo, G. Kumar, G. Pollock, III, H. Carruthers, D. Chaudhary and J. Beasley, Synthesis, 2010, 1386 CrossRef CAS . See also ref. 10.
- Products 6a, 6b, 11i, 11p and 17 were characterized by X-ray crystallography; see ESI† for details.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization. CCDC reference numbers 828268–828272. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1sc00506e |
| This journal is © The Royal Society of Chemistry 2011 |
