Highly efficient CdTe/CdS quantum dot sensitized solar cells fabricated by a one-step linker assisted chemical bath deposition†
Xiao-Yun
Yu
,
Bing-Xin
Lei
,
Dai-Bin
Kuang
* and
Cheng-Yong
Su
MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, KLGHEI of Environment and Energy Chemistry, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou, 510275, P. R. China. E-mail: kuangdb@mail.sysu.edu.cn; Fax: (+)86-20-8411-3015
First published on 17th May 2011
Abstract
We report on an interesting and efficient one-step linker assisted chemical bath deposition method to synthesise CdTe or CdTe/CdS quantum dot sensitized TiO2 photoelectrodes. The CdTe or CdTe/CdS core/shell quantum dots with different size and structure can be easily obtained by controlling the hydrothermal temperature. The QDs are covalently linked to TiO2 nanocrystallites by thioglycolic acid (TGA) bifunctional molecule which also acts as stabilizer and sulfur source in this one-step fabrication. In this sensitized electrode, CdTe has higher light absorptivity while the CdS shell plays a crucial role in the sensitive CdTe QDs protection and photo-generated charges separation. Both effects push the power conversion efficiency of the quantum dot sensitized solar cells (QDSSCs) up to 3.8% and 5.25% under AM 1.5 G one sun (100 mW cm−2) and 0.12 sun illumination, respectively.
Introduction
The superior photophysical and photochemical properties (e.g. tuneable band gap, and high extinction coefficients)1 of semiconductor quantum dots (QDs) offer attractive advantages as light absorbers for the next-generation solar cells.2 Moreover, the recent demonstration of hot electrons and/or multiple exciton generation by impact ionization3 for QDs could push the thermodynamic efficiency up to 44%, beyond the Shockley–Queisser limit.4 Despite the advantages known for QDs, the photovoltaic performance of quantum dot sensitized solar cells (QDSSCs) still lags behind that of dye-sensitized solar cells (DSSCs).Nowadays two typical approaches have been developed to fabricate QD sensitized TiO2 electrodes for QDSSCs: (1) direct synthesis of QDs on TiO2 substrate using chemical bath deposition (CBD),5 or successive ionic layer adsorption and reaction (SILAR) method;6 (2) adsorption of the pre-synthesized colloidal QDs onto the TiO2 surface by direct adsorption (DA)7 or linker assisted adsorption (LA) using bifunctional molecules such as mercaptopropionic acid (MPA).8 Although it is difficult to control the size and size distribution of the QDs using the first method, satisfying cell efficiency can be obtained because of the direct contact between QDs and TiO2 nanocrystallines.9 For the second approach, the efficiency of the QDSSCs was generally lower than 2% due to the very low coverage and/or the poor electronic transport properties of the bifunctional linker.10 Overall, a facile fabrication of QD-sensitized electrodes with good control of QDs size and high efficiency in solar cells is highly desirable.
Among all the QDs utilized in QDSSCs, such as II–VI (CdS, CdSe, CdTe),11 IV–VI (PbS, PbSe),12 and III–V (InP, InAs),13CdSe and CdS are the most efficient and widely used.14 As is known, bulk CdTe has an ideal band gap of 1.56 eV and a relatively positive conductive band edge of −3.70 eV.15 This implies that the light-absorption of CdTe would extend to the near infrared region and the electron injection rate from CdTe QDs to TiO2 would be faster compared to CdSe QDs of the same size. However, the highest power conversion efficiency of CdTe based QDSSCs is only 2.02%,15 prepared by LA method. The limitation comes from the labile Te precursors such as NaHTe or H2Te, which make it impossible to fabricate the QD-sensitized electrodes by the CBD or SILAR method. In addition, CdTe QDs are known to be readily oxidized which seriously diminish the cell efficiency.
In this study, we develop a one-step strategy combining the linker-assisted adsorption and chemical bath deposition methods to fabricate CdTe/CdS QD-sensitized TiO2 electrodes, which is denoted as linker assisted chemical bath deposition (LACBD). As illustrated in Scheme 1, TiO2 film was immersed into an autoclave containing NaHTe, Cd(CH3COO)2, NaOH, thioglycolic acid (TGA) and water. During the hydrothermal reaction process, the formation of QDs and their linking to TiO2 occur simultaneously. The CdTe or core/shell CdTe/CdS QD-sensitized TiO2 electrode can be easily prepared by varying the reaction temperature, and directly utilized in solar cell application without further treatment. The maximum power conversion efficiency (η) of QDSSCs reaches 3.8% under 1 sun illumination, and a value of 5.25% can be obtained under low light intensity (0.12 sun) irradiation, using polysulfide electrolyte.
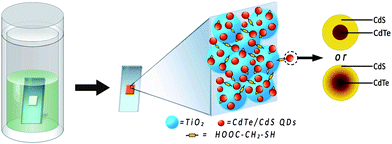 | ||
| Scheme 1 Preparation process and structure of CdTe/CdS core/shell QDs and QD-sensitized TiO2 electrodes by linker assisted chemical bath deposition. | ||
Results and discussion
Fig. 1a shows that the diffraction pattern of TGA capped QDs prepared at 80 °C can be indexed as zinc blende CdTe (JCPDS No. 65–1046). However, the XRD peaks of TGA capped QDs prepared at higher temperature (e.g. 120 or 160 °C) shift to higher angles, locating between the standard zinc blende CdTe and CdS, implying the formation of CdS.16 In addition, the selected-area electron diffraction (SAED) of QDs prepared at 160 °C was also carried out (inset in Fig. S1, ESI†). The (111), (220) and (311) planes of zinc blend CdTe can be clearly distinguished. When the reaction temperature further increases to 200 °C, the XRD pattern shows that large CdTexS1−x nanoparticles were formed with a wurtzite crystal structure. As an example, the TEM images of the CdTe/CdS QD-sensitized TiO2 films prepared at 160 °C are shown in Fig. 1b. It can be clearly seen that a number of QDs (about 6 nm in size) were homogeneously covered onto the surface of TiO2 nanocrystallines. A high-resolution TEM image (inset in Fig. 1b) shows the crystalline lattice of the QDs. TEM measurements of the QDs fabricated at different hydrothermal temperatures are further shown in Fig. S1–4 (ESI†) from which we can conclude that the size (3.1, 4.8, 6.1 nm, respectively) and the crystallinity of QDs increase with the increasing hydrothermal temperature from 80 to 120 to 160 °C. The obvious red shift of onset position in UV-vis absorption spectra (Fig. S5, ESI†) of the QDs further confirm the quantum confinement. The band gap of CdTe or CdTe/CdS can be extracted as 2.20, 1.96 and 1.66 eV for 80, 120 and 160 °C nanoparticles, respectively. Uneven CdTexS1−x alloy nanoparticles, with a band gap of 1.88 eV and 5–50 nm size are observed for the sample prepared at 200 °C.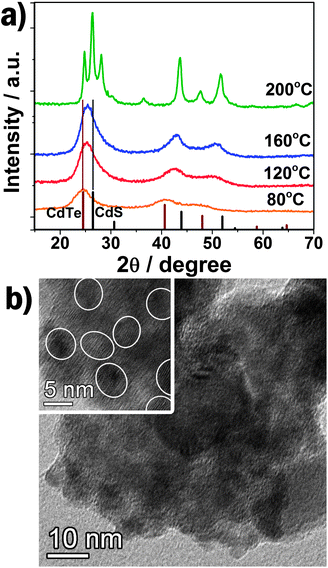 | ||
| Fig. 1 (a) The XRD patterns of QDs and nanoparticles prepared at different hydrothermal temperature with relative intensity of standard zinc blende CdTe and CdS. (b) TEM image of QD-sensitized TiO2. Inset: HRTEM image. | ||
The FTIR spectra of the free TGA, TiO2, CdTe/CdS QDs (prepared at 160 °C) and QDs-TGA-TiO2 are shown in Fig. 2. The characteristic peaks of TGA are observed at 1583 cm−1 (COO asymmetric stretch vibrations) and 1388 cm−1 (CH2 flexural vibrations) for the QDs and QDs-TGA-TiO2, indicating the presence of TGA capping molecule on the QDs and its successful anchoring onto the TiO2 surface. Moreover, the S–H stretching vibration (2565 cm−1), which can be clearly seen in pure TGA, is absent in the spectra of QDs and QDs-TGA-TiO2. This implies the thiol group of TGA combines onto the surface of the QDs through a Cd–S bond.17 On the other hand, the QDs-TiO2 film prepared at 200 °C shows a similar FTIR spectrum as bare TiO2, which gives evidence of complete decomposition of organic TGA during the reaction process.
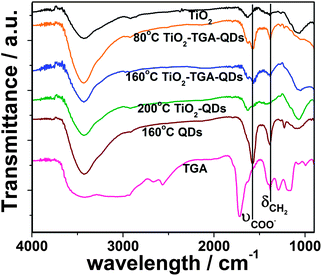 | ||
| Fig. 2 FTIR spectra of bare TiO2, free TGA, CdTe/CdS core/shell QDs prepared at 160 °C, and QD-sensitized electrodes prepared at different temperature. | ||
To investigate the structure of QDs synthesized at different hydrothermal temperature, XPS measurements were carried out. Fig. 3a displays the Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3d spectra of the QDs. The peak at 576.3 eV, which corresponds to the Te
3d spectra of the QDs. The peak at 576.3 eV, which corresponds to the Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3d3/2 level in TeO2, observed for QDs prepared at 50 and 80 °C, is mainly ascribed to the oxidation of CdTe in the open air. However, it disappears for the samples prepared at 120 and 160 °C, implying the stability of CdTe/CdS QDs is much higher than that of individual CdTe QDs, strongly supported the formation of CdS shell on the surface of QDs. A recent report also presents a similar CdTe/CdS core/thick-shell structure by employing MPA as capping agent via a time-consuming two-step method.16b In our experiment, the colour of the CdTe-TGA-TiO2 electrode prepared at 50 °C changes from orange to dark brown in a few seconds after removal from the autoclave, while the electrode prepared at 120 or 160 °C shows no change even after several months. Here, the CdS shell on the CdTe core (synthesized at 120 or 160 °C) plays a critical role in protecting the sensitive CdTe QDs from being oxidized. However, the sample prepared at 200 °C also shows the 3d3/2TeO2 level, which is attributed to the formation of a kind of CdTexS1−x alloy (Fig. 1a, XRD). Further evidence comes from XPS S
3d3/2 level in TeO2, observed for QDs prepared at 50 and 80 °C, is mainly ascribed to the oxidation of CdTe in the open air. However, it disappears for the samples prepared at 120 and 160 °C, implying the stability of CdTe/CdS QDs is much higher than that of individual CdTe QDs, strongly supported the formation of CdS shell on the surface of QDs. A recent report also presents a similar CdTe/CdS core/thick-shell structure by employing MPA as capping agent via a time-consuming two-step method.16b In our experiment, the colour of the CdTe-TGA-TiO2 electrode prepared at 50 °C changes from orange to dark brown in a few seconds after removal from the autoclave, while the electrode prepared at 120 or 160 °C shows no change even after several months. Here, the CdS shell on the CdTe core (synthesized at 120 or 160 °C) plays a critical role in protecting the sensitive CdTe QDs from being oxidized. However, the sample prepared at 200 °C also shows the 3d3/2TeO2 level, which is attributed to the formation of a kind of CdTexS1−x alloy (Fig. 1a, XRD). Further evidence comes from XPS S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2p spectra presented in Fig. 3b. The doublet at 161.6 eV (S
2p spectra presented in Fig. 3b. The doublet at 161.6 eV (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2p3/2) and 162.8 eV (S
2p3/2) and 162.8 eV (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2p1/2) corresponds to S of CdS, and that at 162.3 eV (S
2p1/2) corresponds to S of CdS, and that at 162.3 eV (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2p3/2) and 163.5 eV (S
2p3/2) and 163.5 eV (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2p1/2) is from S of the thiol group which anchors to the QDs. The QDs prepared at 50 °C show only one S doublet of the thiol group, revealing that pure CdTe QDs capped with TGA are obtained. The ratio of S in CdS increases with the reaction temperature (from 80–160 °C), implying the formation of a CdS shell. Furthermore, the peak of S for the thiol group shifts to higher binding energy for samples prepared at 120 or 160 °C, which can be ascribed to the anchoring of the thiol group to the CdS shell. For the CdTexS1−x nanoparticles prepared at 200 °C, no thiol group doublet appears because of the complete decomposition of TGA.
2p1/2) is from S of the thiol group which anchors to the QDs. The QDs prepared at 50 °C show only one S doublet of the thiol group, revealing that pure CdTe QDs capped with TGA are obtained. The ratio of S in CdS increases with the reaction temperature (from 80–160 °C), implying the formation of a CdS shell. Furthermore, the peak of S for the thiol group shifts to higher binding energy for samples prepared at 120 or 160 °C, which can be ascribed to the anchoring of the thiol group to the CdS shell. For the CdTexS1−x nanoparticles prepared at 200 °C, no thiol group doublet appears because of the complete decomposition of TGA.
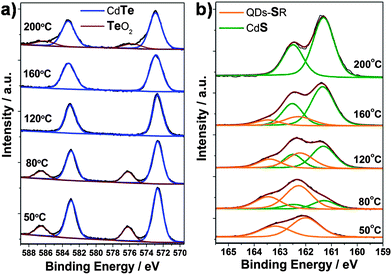 | ||
Fig. 3 (a) Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3d and (b) S 3d and (b) S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2p XPS spectra of CdTe or CdTe/CdS QDs prepared at different reaction temperature. 2p XPS spectra of CdTe or CdTe/CdS QDs prepared at different reaction temperature. | ||
According to the XPS analysis, one can conclude that TGA-capped CdTe QDs and naked CdTexS1−x alloy nanoparticles are formed at low (50 °C) and extreme high hydrothermal temperature (200 °C), respectively. At 80 °C, CdS begins to deposit on the surface of CdTe QDs with a quantity of CdTe remaining exposed. Considering the CdTe crystal nucleus formed before hydrothermal reaction, CdTe/CdS core/shell or CdTe/CdTe–CdS/CdS core/transition/shell structured QDs can be obtained (as shown in Scheme 1) for hydrothermal temperatures between 120 and 160 °C. In the present system, the TGA molecule plays three important roles in the formation of QD-sensitized TiO2 electrode: (1) stabilizer which controls the formation of QDs, the negatively charged TGA molecules wrap up the QDs to prevent them from aggregation; (2) linker which links the QDs (by the thiol group) onto the TiO2 surface (by the carboxyl); (3) a sulfur source since the decomposition of TGA triggers the formation of the CdS shell resulting in the core/shell structure, which also enhances the stability of the QDs in the open air.
The UV-vis absorption spectra of QD-sensitized TiO2 films shown in Fig. 4a demonstrate the absorbance increases with increasing hydrothermal temperature (from 80 to 160 °C) accompanied by a red-shift of the absorption peak and onset position, which implies the larger size and higher loading of CdTe/CdS QDs on the TiO2 surface. The higher absorption value of the visible light and wide absorption area of QD-sensitized TiO2 films with increasing hydrothermal temperature will be significant for the enhancement of the photovoltaic performance of solar cells.
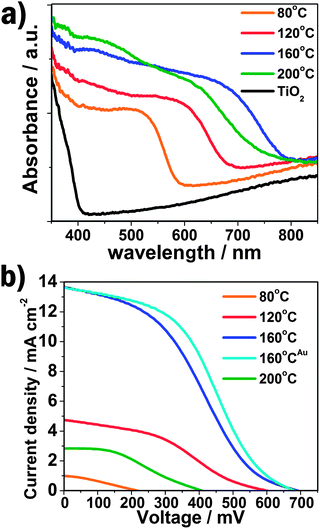 | ||
| Fig. 4 (a) The UV-vis absorption spectra of QD-sensitized TiO2 electrodes prepared under different reaction temperature; (b) I–V curves of QD- or nanoparticle-sensitized solar cells. The superscript Au represents the QDSSC using Au-FTO as counter electrode. | ||
The current–voltage (I–V) characterizations of the QDSSCs by employing a polysulfide electrolyte are shown in Fig. 4b, and the detailed photovoltaic parameters are summarized in Table 1. The JSC and VOC is quite low for the CdTe QDSSCs prepared at 80 °C, resulting in a very poor photovoltaic performance (0.06%), which is due to the limited light absorption and oxidation of CdTe QDs. The JSC and VOC significantly increase with the increasing hydrothermal temperature, which can be ascribed to the larger size and higher loading of CdTe/CdS core/shell QDs on the TiO2 nanocrystalline surface, increasing the light absorption, as demonstrated by the above UV-vis absorption results. Consequently, the power conversion efficiency (η) of the QDSSCs is obviously enhanced. A maximum photovoltaic performance of 3.28% is obtained for the CdTe/CdS core/shell QD-sensitized TiO2 photoelectrode prepared at 160 °C.
| T/°C | J SC/mA cm−2 | V OC/mV | η (%) | FF |
|---|---|---|---|---|
| a QDSSC using Au-FTO as counter electrode. | ||||
| 80 | 1.00 | 218 | 0.06 | 0.30 |
| 120 | 4.74 | 600 | 1.03 | 0.36 |
| 160 | 13.68 | 692 | 3.28 | 0.35 |
| 160a | 13.60 | 682 | 3.80 | 0.41 |
| 200 | 2.84 | 408 | 0.42 | 0.36 |
Besides, the noticeable improvement in JSC and VOC also reveals that the CdTe/CdS core/shell QDs structure exhibits a critical effect in the superior photoelectric property of the QDSSC. The band gap and conductive band edges of TiO2, CdS and CdTe in bulk are 3.2, 2.24, 1.56 eV and −4.21, −3.98, −3.70 eV, respectively.15,18 A step-like band edge structure is observed in the core/shell QD-sensitized TiO2 electrode, similar to the TiO2-CdS-CdSe band structure which has been discussed before.14,19 The energy band segregates the hole to the core and electron to the shell thus offering a high driving force for electron injection from QDs to TiO2. Moreover, CdS has a much lower oxidation potential than CdTe, resulting in low photo-oxidative degradation and surface defects.20 The two effects cooperatively lead to a significant reducing of carrier recombination and hence offer outstanding photovoltaic performance of the CdTe/CdS core/shell QD-sensitized solar cells. As for the electrode prepared at 200 °C, the JSC, VOC, FF and η decrease obviously since the overlarge size of alloy nanocrystals accumulate on the TiO2 surface which increase the photogenerated electron transport distance and the recombination of carriers.
According to the previous study, the Pt counter electrode is supposed to have a low surface activity and conductivity for QDSSCs with polysulfur electrolyte, due to the strong adsorption of the sulfur-containing compound. To overcome this drawback an Au electrode was successfully introduced with an obvious enhancement of the solar cell efficiency.14,21 Here, for the CdTe/CdS sensitized TiO2 electrode prepared at 160 °C, the FF and η of QDSSC increases to 0.41 and 3.80%, respectively, when using Au counter electrode. The incident-photon-to-current conversion efficiency (IPCE) value reaches 53% at 520 nm, as shown in Fig. 5. Additionally, the IPCE is over 40% from 400 to 670 nm, and the onset position is located at nearly 800 nm, fitting well with the UV-vis absorption spectrum, which indicates the significant wide visible light absorption compared to other kinds of QDs (CdS, CdSeetc) utilized in QDSSCs. Furthermore, the power conversion efficiency (see Fig. S6 and Table S1 in ESI†) increases from 3.91, 4.32 to 5.25% under illuminations of 0.52 sun, 0.35 sun and 0.12 sun, respectively, offering a potential design strategy of highly efficient QDSSCs.
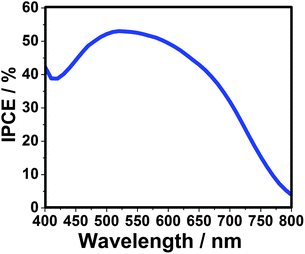 | ||
| Fig. 5 Incident-photon-to-current conversion efficiency (IPCE) curve of QDSSC-based on CdTe/CdS core/shell quantum dots. The sensitized electrode is prepared at 160 °C and using Au-FTO as counter electrode. | ||
Conclusions
In summary, we have demonstrated a one-step linker-assisted chemical bath deposition method to prepare CdTe/CdS core/shell QD-sensitized TiO2 electrodes. In the presence of TGA, the size and structure of the QDs can be easily controlled by varying the hydrothermal temperature. The step-like band structure provides advantages of separating photogenerated charges and anti-oxidation protection. Thus the power conversion efficiency of CdTe based QDSSCs was raised to 3.8 and 5.25% under 1 and 0.12 sun illumination by employing Au counter electrode and polysulfide electrolyte, which is the highest observed for linker method- and/or CdTe-based QDSSCs.Experimental section
Fabrication of QD-sensitized TiO2 photoelectrode
1 ml NaHTe solution (prepared by dissolving 1 mmol tellurium powder and 3 mmol NaBH4 in 5 ml of distilled water) was slowly injected into a solution containing 6 mmol thioglycolic acid (TGA), 2 mmol Cd(CH3COO)2, 14 mmol NaOH and 40 ml water, to form the CdTe crystal nucleus. Then a screen printed P25 (Degussa) film (15 μm in thickness) prepared according to the previous report22 was immersed into the above solution in a Teflon-lined stainless steel autoclave. The sealed autoclave was put into an oven with a certain temperature (50–200 °C). When the 12 h reaction was finished, the white TiO2 films were already changed to a different colour under various hydrothermal temperature. Meanwhile, homogeneous solutions (50–120 °C) or suspensions (160 °C) of individual QDs can be obtained, while insoluble precipitation was generated in 200 °C reaction.Fabrication of QD-sensitized solar cells
The resulting TiO2 films above can be directly used as photoanodes to assemble QDSSCs, which were sandwiched together with a Pt or Au coated fluorine-doped tin oxide (FTO) glass counter electrode. The electrolyte contains 1 M Na2S, 1 M sulfur and 0.1 M NaOH in methanol–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). The active area of the QD-sensitized TiO2 film was about 0.152 cm2.
3, v/v). The active area of the QD-sensitized TiO2 film was about 0.152 cm2.
Characterizations
The as-prepared CdTe or CdTe/CdS QDs were characterized by a Bruker D8 Advance X-ray diffractometer with Cu-Kα radiation (λ = 0.15418 nm). Transmission electron microscopy (TEM) and the selected-area electron diffraction (SAED) of QDs or QD-sensitized TiO2 electrode were carried out by JEM2010-HR. XPS analysis was measured by an X-ray photoelectron spectroscopy ESCA (Thermo Fisher Scientific, ESCALAB 250, Mono Al Kα source 1486.6eV). The UV-visible absorption and FT-IR spectra were measured with a UV-Vis-NIR spectrophotometer (Shimadzu UV-3150) and FT-IR Analyzer (Nicolet/Nexus 670), respectively. The TiO2 film thickness was measured by using a profilometer (Ambios, XP-1). The current–voltage characteristics were performed using a Keithley 2400 source meter under simulated AM 1.5 G illumination (100 mW cm−2) provided by a solar simulator (Oriel, Model: 91192). A 1000 W Xenon arc lamp (Oriel, Model: 6271) served as a light source and its incident-light intensity was calibrated with a NREL-calibrated Si solar cell equipped with a optical filter to approximate AM 1.5 G one sun light intensity before each experiment. The incident-photon-to-current conversion efficiency (IPCE) was recorded on a Keithley 2000 multimeter under the illumination of a 150 W tungsten lamp with a monochrometer (Spectral Product DK240).Acknowledgements
The authors acknowledge the National Natural Science Foundation of China (20873183, 21073239, U0934003), the Fundamental Research Funds for the Central Universities, Research Fund for the Doctoral Program of Higher Education (20100171110014).Notes and references
- (a) W. W. Yu, L. H. Qu, W. Z. Guo and X. G. Peng, Chem. Mater., 2003, 15, 2854 CrossRef CAS; (b) P. V. Kamat, J. Phys. Chem. C, 2008, 112, 18737 CAS; (c) S. F. Wuister, A. van Houselt, C. D. M. Donega, D. Vanmaekelbergh and A. Meijerink, Angew. Chem., Int. Ed., 2004, 43, 3029 CrossRef CAS.
- S. Rühle, M. Shalom and A. Zaban, ChemPhysChem, 2010, 11, 2290 CrossRef.
- J. B. Sambur, T. Novet and B. A. Parkinson, Science, 2010, 330, 63 CrossRef CAS.
- V. I. Klimov, J. Phys. Chem. B, 2006, 110, 16827 CrossRef CAS.
- O. Niitsoo, S. K. Sarkar, C. Pejoux, S. Rühle, D. Cahen and G. Hodes, J. Photochem. Photobiol., A, 2006, 181, 306 CrossRef CAS.
- L. J. Diguna, Q. Shen, J. Kobayashi and T. Toyoda, Appl. Phys. Lett., 2007, 91, 023116 CrossRef.
- N. Guijarro, T. Lana-Villarreal, I. Mora-Seró, J. Bisquert and R. Gómez, J. Phys. Chem. C, 2009, 113, 4208 CrossRef CAS.
- (a) H. M. Chen, C. K. Chen, Y. C. Chang, C. W. Tsai, R. S. Liu, S. F. Hu, W. S. Chang and K. H. Chen, Angew. Chem., Int. Ed., 2010, 49, 5966 CAS; (b) I. Robel, V. Subramanian, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2006, 128, 2385 CrossRef CAS.
- I. Mora-Seró, S. Giménez, F. Fabregat-Santiago, R. Gómez, Q. Shen, T. Toyoda and J. Bisquert, Acc. Chem. Res., 2009, 42, 1848 CrossRef CAS.
- D. F. Watson, J. Phys. Chem. Lett., 2010, 1, 2299 Search PubMed.
- (a) J. H. Bang and P. V. Kamat, ACS Nano, 2009, 3, 1467 CrossRef CAS; (b) A. Kongkanand, K. Tvrdy, K. Takechi, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2008, 130, 4007 CrossRef CAS.
- (a) R. Plass, S. Pelet, J. Krueger, M. Grätzel and U. Bach, J. Phys. Chem. B, 2002, 106, 7578 CrossRef CAS; (b) H. J. Lee, P. Chen, S. J. Moon, F. Sauvage, K. Sivula, T. Bessho, D. R. Gamelin, P. Comte, S. M. Zakeeruddin, S. II Seok, M. Grätzel and M. K. Nazeeruddin, Langmuir, 2009, 25, 7602 CrossRef CAS.
- (a) P. R. Yu, K. Zhu, A. G. Norman, S. Ferrere, A. J. Frank and A. J. Nozik, J. Phys. Chem. B, 2006, 110, 25451 CrossRef CAS; (b) R. B. Laghumavarapu, M. El-Emawy, N. Nuntawong, A. Moscho, L. F. Lester and D. L. Huffaker, Appl. Phys. Lett., 2007, 91, 243115 CrossRef.
- Y. L. Lee and Y. S. Lo, Adv. Funct. Mater., 2009, 19, 604 CrossRef.
- G. Y. Lan, Z. S. Yang, Y. W. Lin, Z. H. Lin, H. Y. Liao and H. T. Chang, J. Mater. Chem., 2009, 19, 2349 RSC.
- (a) H. B. Bao, Y. J. Gong, Z. Li and M. Y. Gao, Chem. Mater., 2004, 16, 3853 CrossRef CAS; (b) Z. T. Deng, O. Schulz, S. Lin, B. Q. Ding, X. W. Liu, X. X. Wei, R. Ros, H. Yan and Y. Liu, J. Am. Chem. Soc., 2010, 132, 5592 CrossRef CAS.
- R. S. Dibbell, G. R. Soja, R. M. Hoth and D. F. Watson, Langmuir, 2007, 23, 3432 CrossRef CAS.
- M. Grätzel, Nature, 2001, 414, 338 CrossRef CAS.
- Y. L. Lee, B. M. Huang and H. T. Chien, Chem. Mater., 2008, 20, 6903 CrossRef CAS.
- A. M. Smith and S. Nie, Acc. Chem. Res., 2010, 43, 190 CrossRef CAS.
- S. Giménez, I. Mora-Seró, L. Macor, N. Guijarro, T. Lana-Villarreal, R. Gómez, L. J. Diguna, Q. Shen, T. Toyoda and J. Bisquert, Nanotechnology, 2009, 20, 295204 CrossRef.
- B. X. Lei, W. J. Fang, Y. F. Hou, J. Y. Liao, D. B. Kuang and C. Y. Su, J. Photochem. Photobiol., A, 2010, 216, 8 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images and SAED pattern of QDs, the UV-vis absorption spectra of individual QDs, the I–V curves and detailed parameters of CdTe/CdS QDSSCs under various low light densities. See DOI: 10.1039/c1sc00144b |
| This journal is © The Royal Society of Chemistry 2011 |
