Kinetics-driven high power Li-ion battery with a-Si/NiSix core-shell nanowire anodes†
Kibum
Kang
a,
Kyeongse
Song
c,
Hoseok
Heo
b,
Sunyoung
Yoo
a,
Gil-Sung
Kim
a,
Geunhee
Lee
a,
Yong-Mook
Kang
*c and
Moon-Ho
Jo
*ab
aDepartment of Materials Science and Engineering, Pohang University of Science and Technology (POSTECH), San 31, Hyoja-Dong, Nam Gu, Pohang, Gyungbuk 790-784, Korea. E-mail: mhjo@postech.ac.kr; Fax: +82-54-279-2399; Tel: +82-54-279-2158
bDivision of Advanced Materials Science, Pohang University of Science and Technology (POSTECH), San 31, Hyoja-Dong, Nam Gu, Pohang, Gyungbuk 790-784, Korea
cDivision of Advanced Materials Engineering, Kongju National University, 275 Budae-Dong, Cheonan, Chungnam 330-717, Korea. E-mail: dake1234@kongju.ac.kr; Tel: +82-41-521-9378
First published on 25th March 2011
Abstract
We report amorphous-Si nanowire shell anodes, supported by NiSixnanowire cores, by catalyst-free two-step SiH4 chemical vapor deposition, where the metallic core acts as a mechanical supporter and a kinetically unlimited charge supplier. We have achieved highly reversible capacitance of over 3000 mAh g−1 even at a 2C rate, with stable cyclic retention which stems from the altered electrochemical reactions with relatively small volume expansion routes by a kinetic effect.
Introduction
Anode architectures, particularly when they are three-dimensional at the nanometre scale, are closely related with cell performances in Li-ion batteries.1–6 Therein, the achievable electrochemical capacity and the power characteristics are inherently determined by a series of phase transitions involved in the anode during lithiation/delithiation. The electrochemical Li–Si system forms various Li-rich LixSi intermetallics (0 ≤ x ≤ 4.4), correspondingly symptomatic of the largest known gravimetric charge capacity of up to 4200 mAh g−1 in the bulk limit, thus can represent an attractive anodic system for the high capacity Li-ion battery.1,3–5However, the system undergoes notoriously large volume-changes up to 400% during the cyclic lithiation and delithiation, resulting in poor cyclic retention in its cell capacity and output power.1,3–5 It is suggested that this severe anode pulverization upon the cyclic volume changes can be alleviated by adopting one-dimensional Si nanocrystals, such as Si nanowires (NWs), based on the promises of their affordable accommodation of large volume-changes and the efficient charge collection capability.4,5,7–11 Yet, monolithic single-crystalline Si NWs exhibit cyclic degradation in their cell capacity particularly at the high charging/discharging rate, thus limiting their integration into the high power cells.5,12–18
Here, we report amorphous-Si (a-Si) shell anodes, supported by NiSix NW cores, by a catalyst-free two-step SiH4 chemical vapor deposition, where the metallic core acts as a mechanical supporter and a kinetically unlimited charge supplier. Spontaneous silicide NWs, such as NiSix, FeSi, CoSi, TaSi219–27 NWs, have been recently investigated, and among others NiSix NWs are of particular interest because their growth can be easily integrated with silicon processing technology. Besides, NiSix exhibits the lowest resistivity and makes good contacts with Si.25–27 In this regard, we designed the core-shell NW anode architecture in this work. We have achieved the highly reversible capacity over 3000 mAh g−1 even at 2C rate, with stable cyclic retention which stems from the altered electrochemical reactions with relatively small volume expansion routes by a kinetic effect.
Results and discussion
a-Si NW shells, supported by metallic NiSix NW cores (a-Si/NiSix NW), were grown by a simple two-step SiH4 CVD process: (i) the self-catalytic NiSix NWs were grown on Ni thin films, (ii) followed by a-Si shells deposition on the NiSix NWs, as illustrated in Fig. 1(a). We start off the NiSix NW synthesis by thermal evaporation of Ni films on Ag-coated stainless steel (SUS) substrates of a circular shape disk in 15mm diameter. Ag acts as a diffusion barrier of Fe from SUS into the Ni films. The Ni/Ag/SUS substrates were annealed with oxygen at 400 °C, and then reacted with 50 Torr of SiH4 (10% diluted in H2) at 420 °C, as described in Ref. 26,27. The a-Si shell deposition was conducted by using thermal decomposition of SiH4 (10% diluted in H2) at 550 °C for 30 min. Here we note that a-Si shells are formed, when NiSix NWs are oxygen-exposed prior to the SiH4 flows, whereas the crystalline Si shell (c-Si) was formed otherwise, as shown in Fig. S1.† We also prepared a-Si shell NWs on the insulating c-Si core NWs, and describe a series of comparative cyclic cell performances in greater details,28 and discuss them around the roles of the metallic core for altered electrochemical reactions.29,30![(In color) (a) The schematics of the NW heterostructure growth in this study. (b) TEM image of an individual a-Si/NiSix NW. The inset shows a plan-view SEM image of a-Si /NiSix NWs grown on SUS substrate. (c) HRTEM image of an individual a-Si/NiSix NW at the core region and the corresponding FFT-DP along the [1–10] zone axis (inset). (d) HRTEM image of an individual a-Si/NiSix NW at the shell region and the corresponding FFT-DP (inset). (e) HAADF image of a-Si/NiSix NW. (f and g) EDX elemental mapping images of Si (f) and Ni (g) in (e).](/image/article/2011/SC/c0sc00628a/c0sc00628a-f1.gif) | ||
| Fig. 1 (In color) (a) The schematics of the NW heterostructure growth in this study. (b) TEM image of an individual a-Si/NiSix NW. The inset shows a plan-view SEM image of a-Si /NiSix NWs grown on SUS substrate. (c) HRTEM image of an individual a-Si/NiSix NW at the core region and the corresponding FFT-DP along the [1–10] zone axis (inset). (d) HRTEM image of an individual a-Si/NiSix NW at the shell region and the corresponding FFT-DP (inset). (e) HAADF image of a-Si/NiSix NW. (f and g) EDX elemental mapping images of Si (f) and Ni (g) in (e). | ||
Fig. 1(b) shows the representative microstructures of a-Si/NiSix NWs by transmission electron microscope (TEM), where the average diameter of a-Si/NiSix NW is 190 nm with the 20 nm thick NiSix core NWs. Scanning electron microscope (SEM) images in the inset illustrate that the shell NWs were conformally deposited onto the whole NWs in the substrate – see also Fig. S2 in Supporting Information.†High-resolution TEM (HRTEM) images and corresponding fast fourier transform diffraction patterns (FFT-DP) in Fig. 1(c) and 1(d) confirm that Si/NiSix NW features amorphous Si NW shells and single-crystalline NiSi2 NW cores.31 High-angle annular dark field (HAADF) images, where the intensity is roughly proportional to square of atomic number, and energy dispersive X-ray spectroscopy (EDX) mapping images in Fig. 1(e), (f) and (g) also demonstrate that core/shell NW structures are clearly discernable, and the elemental distribution of Ni is locally limited in the core region within the instrumental resolution.
The a-Si/NiSix NW anodes were incorporated into a coin-type half cell (CR2016 coin-type). The cell uses Li metal foils as the counter and the reference electrode, and 1M LiPF6 dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (by volume, provided by Techono. Semichem. Co.) mixture of ethylene carbonate (EC) and diethyl carbonate (DEC) as the electrolyte. The cell assembly was carried out in an Ar-filled glove box with less than 0.1 ppm each of oxygen and moisture. The charge capacity of the a-Si/NiSix NW cell was measured over the potential range of 0.01 to 2.00 V (vs.Li/Li+) during the initial 50 cycles in the galvanostatic mode. The measured discharge capacity is carefully normalized into the gravimetric capacity, as described in Fig. S3.†
1 (by volume, provided by Techono. Semichem. Co.) mixture of ethylene carbonate (EC) and diethyl carbonate (DEC) as the electrolyte. The cell assembly was carried out in an Ar-filled glove box with less than 0.1 ppm each of oxygen and moisture. The charge capacity of the a-Si/NiSix NW cell was measured over the potential range of 0.01 to 2.00 V (vs.Li/Li+) during the initial 50 cycles in the galvanostatic mode. The measured discharge capacity is carefully normalized into the gravimetric capacity, as described in Fig. S3.†
Fig. 2 shows the cyclic retention of two types of NWs: a-Si/NiSix NW cells and a-Si/c-Si NW cells. It is found that a-Si/NiSix NWs retain almost the 83% of the first discharge capacity after the 50th cycle with the high charge capacity above 3000 mAh g−1. For a check, we tested the bare NiSix NW anodes in the galvanostatic mode; however, we did not observe any apparent potential plateau over the potential range of 0.01 to 3.00 V – also see Fig. S4.† This suggests that the bare NiSix NWs alone are electrochemically inactive in our experimental conditions. We have also tested the rate dependent capacity by charging the cell at a constant rate of 0.1C and discharging it at the various rate from 0.1C to 2C (1C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) 1 h per half-cycle). Fig. 2(b) shows that the discharge capacity of a-Si/NiSix NWs is maintained over 94% of the first discharge capacity, and weakly depends on the C-rate up to 2C. The discharge capacity of a-Si/c-Si NWs, on the other hand, fades significantly and strongly depends on the C-rate. It is obvious that a combination of a-Si shells and metallic NiSix NW cores is responsible for the unprecedented reversible lithiation/delithiation in a-Si shells at the high charge/discharge rate.
1 h per half-cycle). Fig. 2(b) shows that the discharge capacity of a-Si/NiSix NWs is maintained over 94% of the first discharge capacity, and weakly depends on the C-rate up to 2C. The discharge capacity of a-Si/c-Si NWs, on the other hand, fades significantly and strongly depends on the C-rate. It is obvious that a combination of a-Si shells and metallic NiSix NW cores is responsible for the unprecedented reversible lithiation/delithiation in a-Si shells at the high charge/discharge rate.
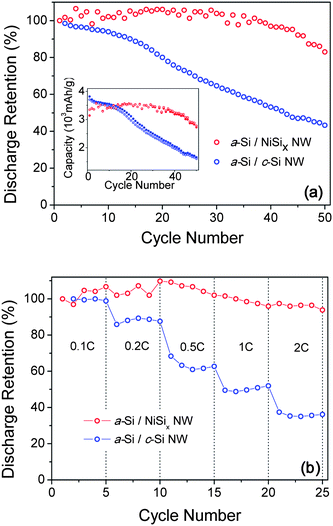 | ||
| Fig. 2 (In color) (a) The discharge capacity retention for the a-Si/NiSix NW (red circles) and the a-Si/c-Si NW (blue circles), respectively. The inset shows the specific capacity for the a-Si/NiSix NW (red close circles-charge and red open circles-discharge) and the a-Si/c-Si NW (blue close circles-charge and blue open circles-discharge), respectively. (b) The rate capability for the a-Si/NiSix NW (red circles) and the a-Si/c-Si NW (blue circles), respectively. | ||
Fig. 3(a–c) present HRTEM and HAADF images of a-Si/c-Si NWs after the 50 cycles, where they become porous and pulverized upon the cyclic lithiation/delithiation. By striking contrast, a-Si/NiSix NWs maintain the initial core/shell NW structures. Apparently, the structural robustness in a-Si/NiSix NWs, guaranteed from a mechanical supporter of NiSix cores, is responsible for the enhanced cyclic retention and rate capability, as shown in Fig. 3(d–f).
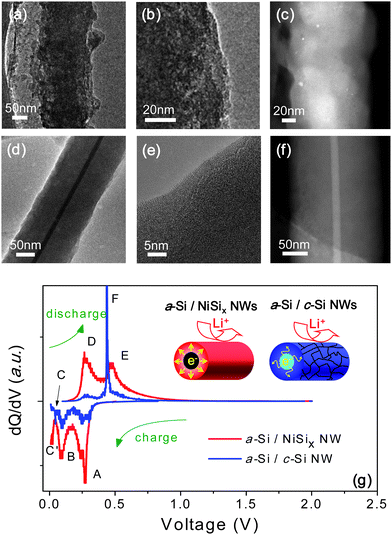 | ||
| Fig. 3 (In color) (a–f) TEM images of individual a-Si/c-Si NW and a-Si/NiSix NW after 50th cycling. (a) Low magnitude TEM image of a-Si/c-Si NW. (b) HRTEM image of a-Si/c-Si NW at the surface. (c) HAADF image of a-Si/c-Si NW. (d) Low magnitude TEM image of a-Si/NiSix NW. (e) HRTEM image of a-Si/NiSix NW at the surface. (f) HAADF image of a-Si/NiSix NW. (g) The differential capacity of the 10th cycle for the a-Si/NiSix NW (red line), the a-Si/c-Si NW (blue line). | ||
In order to further investigate the electrochemical roles of the metallic NiSix cores, we tried to compare the differential capacity plots for a-Si/NiSix NWs with those of a-Si/c-Si NWs, as in Fig. 3(g). After the 1st cycle, which is called the formation or activation process, a-Si/c-Si NWs and a-Si/NiSix NWs display two reduction peaks around 260 mV and 80 mV, as marked by A and B, and they are associated with the formations of intermetallic compounds, LixSi. Reportedly, the 260 mV and 80 mV peaks are assigned to the formation of amorphous LixSi (a-LixSi) from a-Si and c-Si, respectively.32–34 Although other small reduction peaks below 80 mV, marked as C and C′, are not well identified, they are usually assigned to either routes for a-LixSi to a-Lix+ySi (fully lithiated amorphous lithium silicides) transition or a-LixSi to c-LizSi (fully lithiated crystalline lithium silicides) transition. Identification of the fully lithiated phase whether it is a-LixSi to c-LizSi is critical, since the a-LixSi to c-LizSi or vice versa among the series of lithiation/delithiation reactions involves the largest volume changes, which is in turn the major source for the deterioration in the anodic reversibility.34–37 During the discharge process, a-Si/c-Si NWs display the dominant oxidation peak at 450 mV, marked as F, assigned to the dissociation of c-LizSi to a-LixSi or a-Si, indicating that its reduction peak below 80 mV corresponds to the nucleation of a crystalline lithium silicide (c-LizSi).34–36 By contrast, a-Si/NiSix NWs exhibit two dissimilar oxidation peaks between 250 mV and 500 mV, marked as D and E, which are assigned to the dissociation of a-Lix+ySi to a-LixSi or a-Si.32,34,37 This finding can be back-traced to assign another phase transition, C′ during the Li charging to the formation of a-Lix+ySi. In other words, the full lithiation into a-Lix+ySi in a-Si/NiSix, instead of c-LizSi in a-Si/c-Si NWs, alleviates severe physical pulverization and leads to the stable reversibility in the cyclic cell performance. We speculate that kinetically unlimited electron supply from NiSix NW core coupled with the properly absorbed volume change by a-Si shell provides alternative electrochemical reactions of a-Si/NiSix NWs to skip over the nucleation of c-LixSi by a kinetic effect. We measured average resistivity of NiSix NWs and c-Si NWs to be 10−3 Ω·cm, and 103 Ω·cm. It is known that monolithic Si crystals usually undertake temporal local-concentration of Li ions in Si matrices, followed by the rapid and spatially inhomogeneous evolution of fully lithiated crystalline lithium silicides (c-LizSi), which in turn results in severe Si pulverizations. Instead, we argue that uniform distribution of the electrochemical potential in our a-Si/NiSix NWs, which is provided by the metallic NiSix cores is responsible for the reversible reaction route mediated by fully lithiated amorphous lithium silicides.3,32,36
Acknowledgements
M.-H.J. acknowledges the support from the Nano Original & Fundamental Technology R&D Program (2010-0019195), the Priority Research Centers Program (2010-0029711), the Basic Research Program (2010-0001873 and 2010-0017853), the Mid-career Researcher Program (2010-0027627) and the WCU Program (R31-2008-000-10059-0) through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science, and Technology (MEST), and POSCO. Y.-M.K. acknowledges the Converging Research Center Program (2010K001097) and the Basic Science Research Program (20090072972) through the NRF of Korea funded by the MEST.Notes and references
- P. G. Bruce, B. Scrosati and J.-M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS.
- I. Grigoriants, L. Sominski, H. Li, I. Ifrgan, D. Aurbach and A. Gedanken, Chem. Commun., 2005, 7, 921 Search PubMed.
- S.-H. Ng, J. Wang, D. Wexler, K. Konstantinov, Z.-P. Guo and H.-K. Liu, Angew. Chem., Int. Ed., 2006, 45, 6896 CrossRef CAS.
- H. Kim and J. Cho, Nano Lett., 2008, 8, 3688 CrossRef CAS.
- J. R. Szczech and S. Jin, Energy Environ. Sci., 2011, 4, 56 RSC.
- J. R. Dahn, T. Zheng, Y. Liu and J. S. Xue, Science, 1995, 270, 590 CrossRef CAS.
- C. K. Chan, H. Peng, G. Liu, K. Mcilwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31 CrossRef CAS.
- K. Peng, J. Jie, W. Zhang and S.-T. Lee, Appl. Phys. Lett., 2008, 93, 033105 CrossRef.
- H. Kim, B. Han, J. Choo and J. Cho, Angew. Chem., Int. Ed., 2008, 47, 10151 CrossRef CAS.
- J. Graetz, C. C. Ahn, R. Yazami and B. Fultz, Electrochem. Solid-State Lett., 2003, 6, A194 CrossRef CAS.
- C. K. Chan, X. F. Zhang and Y. Cui, Nano Lett., 2008, 8, 307 CrossRef CAS.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353 CrossRef CAS.
- B. Gao, S. Sinha, L. Fleming and O. Zhou, Adv. Mater., 2001, 13, 816 CrossRef CAS.
- L.-F. Cui, Y. Yang, C.-M. Hsu and Y. Cui, Nano Lett., 2009, 9, 3370 CrossRef CAS.
- S. Zhou, X. Liu and D. Wang, Nano Lett., 2010, 10, 860 CrossRef CAS.
- L.-F. Cui, R. Ruffo, C. K. Chan, H. Peng and Y. Cui, Nano Lett., 2009, 9, 491 CrossRef CAS.
- S. Zhang, Z. Du, R. Lin, T. Jiang, G. Liu, X. Wu and D. Weng, Adv. Mater., 2010, 22, 5378 CrossRef CAS.
- T. Song, J. Xia, J.-H. Lee, D. H. Lee, M.-S. Kwon, J.-M. Choi, J. Wu, S. K. Doo, H. Chang, W. I. Park, D. S. Zang, H. Kim, Y. Huang, K.-C. Hwang, J. A. Rogers and U. Paik, Nano Lett., 2010, 10, 1710 CrossRef CAS.
- A. L. Schmitt, M. J. Higgins, J. R. Szczech and S. Jin, J. Mater. Chem., 2010, 20, 223 RSC.
- J. R. Szczech and S. Jin, J. Mater. Chem., 2010, 20, 1375 RSC.
- A. L. Schmitt, M. J. Higgins, D. Schmeisser, F. J. Himpsel and S. Jin, Nano Lett., 2006, 6, 1617 CrossRef CAS.
- Y. Song, A. L. Schmitt and S. Jin, Nano Lett., 2006, 6, 965.
- K. Seo, K. S. K. Varadwaj, P. Monhanty, S. Lee, Y. Jo, M.-H. Jung, J. Kim and B. Kim, Nano. Lett., 1240, 2007, 7 Search PubMed.
- Y.-L. Chueh, M.-T. Ko, L.-J. Chou, L.-J. Chen, C.-S. Wu and C.-D. Chen 2006, 6, p. 1637.
- K. Kang, C.-J. Kim and M.-H. Jo, J. Appl. Phys., 2009, 105, 122407 CrossRef.
- K. Kang, S.-K. Kim, C.-J. Kim and M.-H. Jo, Nano Lett., 2008, 8, 431 CrossRef CAS.
- C.-J. Kim, K. Kang, Y. S. Woo, K.-G. Ryu, H. Moon, J.-M. Kim, D.-S. Zang and M.-H. Jo, Adv. Mater., 2007, 19, 3637 CrossRef CAS.
- K. Kang, H.-S. Lee, D.-W. Han, G.-S. Kim, D.-H. Lee, G. Lee, Y.-M. Kang and M.-H. Jo, Appl. Phys. Lett., 2010, 96, 053110 CrossRef.
- J. Yin, M. Wada, K. Yamamoto, Y. Kitano, S. Tanase and T. J. Sakai, J. Electrochem. Soc., 2006, 153, A472 CrossRef CAS.
- Graetz, C. C. Ahn, R. Yazami and B. Fultz, Electrochem. Solid-State Lett., 2003, 6, A194 CrossRef CAS.
- Crystal structure of Si: diamond cubic, lattice constant: 5.431 Å, space group: Fm3m, JCPDS 43–0989; Crystal structure of NiSi2: diamond cubic, lattice constant: 5.416 Å space group: Fd-3m, JCPDS 27–1402.
- M. N. Obrovac and L. J. Krause, J. Electrochem. Soc., 2007, 154, A103 CrossRef CAS.
- J. P. Maranchi, A. F. Hepp and P. N. Kumta, Electrochem. Solid-State Lett., 2003, 6, A198 CrossRef CAS.
- C. K. Chan, R. Ruffo, S. S. Hong, R. A. Huggins and Y. Cui, J. Power Sources, 2009, 189, 34 CrossRef CAS.
- T. D. Hatchard and J. R. Dahn, J. Electrochem. Soc., 2004, 151, A838 CrossRef CAS.
- Y. M. Kang, S. M. Lee, S. J. Kim, G. J. Jeong, M. S. Sung, W. U. Choi and S. S. Kim, Electrochem. Commun., 2007, 9, 959 CrossRef CAS.
- N. Obrovac and L. Christensen, Electrochem. Solid-State Lett., 2004, 7, A93 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Structure of c-Si/NiSix NW, a photograph of the a-Si/NiSix NW grown on substrate, normalization method for calculating the gravimetric capacity, and current charge/discharge curve for NiSix NW cell in the galvanostatic mode. See DOI: 10.1039/c0sc00628a |
| This journal is © The Royal Society of Chemistry 2011 |
