Solid lipid nanoparticles (SLN) templating of macroporous silica beads†
Andreea
Pasc
*a,
Jean-Luc
Blin
a,
Marie-José
Stébé
a and
Jaafar
Ghanbaja
b
aSRSMC UMR 7565, Nancy-Université, BP 70239, F-54506, Vandoeuvre-lès-Nancy, France. E-mail: andreea.pasc@srsmc.uhp-nancy.fr; Fax: (0033)383684322; Tel: (0033)383684344
bSCMEM, Nancy-University, BP 239, 54506, Vandoeuvre-lès-Nancy, France
First published on 28th September 2011
Abstract
We report the first example of solid lipid nanoparticles (SLN) templating silica. The biocompatible nanoparticles were obtained from cetylpalmitate and Tween 20 via the solvent injection method. The macroporous material consists of silica beads of 0.5–1.5 μm with a hollow morphology, imprinting from the starting SLN, of 50–400 nm in size.
Porous materials design remains an important scientific and technological issue due to their wide range of applications in catalysis,1 sensing,2 sorption or separation3 processes.
Colloidal templating has been widely used to fabricate such materials4 with different shapes and sizes ranging from several nm (micro and mesoporous) to several microns (macroporous). Hierarchical macro–mesostructured silica has been successfully synthesized by the use of self-assembled templates of colloidal spheres5 such as polystyrene (PS), poly(methyl methacrylate) (PMMA) latex spheres, silica spheres,6 and emulsion droplets7 with a narrow size distribution.
Silica is also known to be safe, not only for the environment, but also for the human body within a certain range of administrated dose, approved by US Food and Drug Administration (FDA).8 Therefore, its application field may be extended to biocompatible materials, such as bone substitutes, cements for bone repair and reconstruction, enzyme and cell immobilization, biocatalysis or biosensors9 and, recently, for oral drug delivery.10
In the soft matter field, Solid Lipid Nanoparticles (SLN) appeared very recently as promising drug carriers especially for their potential applications in pharmaceutics.11 Therefore, combining inorganic silica matter with a solid lipid, SLN appears as a straightforward approach for the development of novel hybrid organic–inorganic biocompatible materials.
SLN have not only a limited toxicity, but they can be produced in a cost-effective fashion, by different formulation techniques: high pressure homogenization, emulsification–sonication, microemulsion, double emulsion, solvent emulsification–evaporation, solvent diffusion and solvent injection. The last one was chosen for the synthesis of the SLN reported herein,12 since it presents clear advantages over the existing methods, such as easy handling and fast production process without technically sophisticated equipment. The solvent injection method, similar to solvent diffusion method, is based on lipid precipitation from a dissolved lipid in solution.
The SLN-templated porous material was prepared‡ by adding a silica source (tetramethoxylsilane, TMOS), at neutral pH, on SLN dispersion, and the mixture was kept in an autoclave at 70 °C to allow the hydrolysis/polycondensation of the silica. The inorganic material was obtained after the removal of the organic nanoparticles by ethanol/dichloromethane extraction. The designed strategy synthesis is illustrated in Fig. 1.
 | ||
| Fig. 1 Schematic illustration of SLN formation and templating of macroporous silica beads. | ||
The SLN were prepared† by mixing under stirring, a solution of cetylpalmitate (n-hexadecylpalmitate, NHP) in THF to a hot (65 °C) diluted solution of a biocompatible surfactant, Tween 20. Dynamic light scattering (DLS) measurements and transmission electron microscopy (TEM) micrographs (Fig. 2) revealed that the suspension was composed of spherical (α-form) particles ranging from 50 to 400 nm, centered at 250 nm. The particles were stable for more than 1 month and no gelation was observed, probably due to the absence of polymorphic transformations of the solid lipid during the storage.13
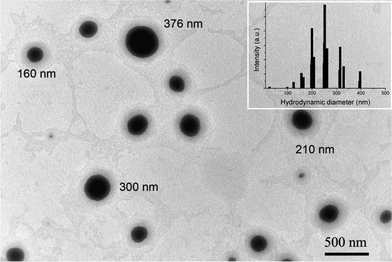 | ||
| Fig. 2 TEM micrograph of prepared SLN (insert: hydrodynamic diameter of SLN as determined by DLS). | ||
Small-angle X-ray scattering (SAXS) investigations (Fig. S1) show that the cetylpalmitate powder is polymorphic whereas the lipid of the SLN is arranged in a lamellar lattice structure, since the X-ray diffractogram exhibits the 100 and 300 peak reflections, the 200 being very low. The corresponding interlayer spacing is 3.9 nm (compared to 4.1 nm found in the literature14) and remains constant in the hybrid organic–inorganic material. The lamellar structure of the SLN was confirmed by TEM analysis (Fig. S3). At 1.5%, the concentration used to prepare the SLN, Tween 20 forms micelles (cmc = 8 × 10−5 M) of around 10 nm in diameter (Fig. S2). However, herein the surfactant is essentially stabilizing the SLN dispersions, and no micelles were observed neither by DLS nor by SAXS measurements.
SAXS data of SLN-free silica material (data not shown) exhibit a broad low angle reflection in the region 4–7 nm, which suggests the existence of unorganized mesopores.
Evidence of the mesoporosity can also be reached from the nitrogen adsorption isotherms (Fig. S4). According to the IUPAC classification, the sample exhibits a type II isotherm, characteristic of macroporous material. The pore size distribution obtained by the BJH method applied to the adsorption branch of the isotherm evidences mesopore sizes of 7 nm. However, the low volume of dV/dD indicates that the proportion of mesopores in the sample is rather weak. The BET specific surface area and the mesopore volume are 50 m2 g−1 and 53 mm3 g−1, respectively. The macroporous network can be observed by SEM analysis. As a matter of fact, silica beads containing spherical macropores of 0.5–1.5 μm in diameter are clearly shown in Fig. 3. The pores are not well ordered and range between 50 and 150 nm. Also, large hollow silica beads were observed and the cavity diameter is around 500 nm. These macropore diameters are of the same order of magnitude as the starting SLN. Deeper insights on the morphology of the material were reached by TEM. The thickest silica external shell was 80 nm and the thinnest silica internal wall was 4 nm (Fig. 4c).
 | ||
| Fig. 3 SEM micrographs of SLN-templating porous silica beads. | ||
 | ||
| Fig. 4 TEM micrographs of SLN-templating porous silica. | ||
Clear evidence of the macroporosity can be reached from mercury intrusion porosimetry measurements§(Fig. 5). The specific surface area of the sample (27.1 m2 g−1) is smaller than the BET surface area but the porous volume is more than 10 times higher (864 mm3 g−1). As indicated in Table 1, the pores smaller than 438 nm are responsible for the surface area of the sample, whereas they represent only 39% of the relative pore volume. The material exhibits a multimodal size distribution: three broad peaks centered at 9, 68 and 450 nm in diameter. The peak at 9 nm could be attributed to mesopores. The increase in the Hg adsorption isotherm above 3 μm could be attributed to inter-particular distances between hollow silica beads. Finally, the peak at 450 nm could correspond to the hollow silica whereas the one at 68 nm corresponds to the secondary, multi cavity structure of some silica beads. Thus, one can conclude that these pores are the imprint of the starting morphology of the nanoparticles and appear as spherical cavities, as it was visualized by scanning and transmission electron microscopy (Fig. 3 and 4).
 | ||
| Fig. 5 Pore size distribution obtained by applying the Washburn equation to the mercury intrusion curves. | ||
| Pore diameter ranges (μm) | Relative volume (mm3 g−1) | Relative surface (m2 g−1) |
|---|---|---|
| 10000–0.438 | 528 (61%) | 1.5 |
| 0.438–0.001 | 336 (39%) | 25.6 |
In conclusion, we report the synthesis of macroporous silicaviaSLN template-directed hydrolysis–polycondensation reactions of TMOS. To the best of our knowledge, this templating route is the first example reported in the literature. Work is underway to tune the size of the SLN in order to control the porosity of the resulting silica and to further organize the inorganic matter at the mesoporous–macroporous scale. SLN-silica matter could be tailored afterwards as drug carriers with a potential application in cosmetics and pharmaceutics, such as oral drug delivery.
The authors would like to thank Amélie Clément (masters student) for her contribution to this work, Mélanie Emo for performing the X-ray measurements, Jonathan Jacoby and Anna May for the nitrogen adsorption–desorption isotherms and Lionel Richaudeau for the mercury intrusion porosimetry analysis.
References
- (a) A. Corma, Chem. Rev., 1997, 97, 2373–2419 CrossRef CAS; (b) J. F. Brown, P. Krajnc and N. R. Cameron, Ind. Eng. Chem. Res., 2005, 44, 8565–8572 CrossRef CAS.
- M. S. Silverstein, H. Tai, A. Sergienko, Y. Lumelsky and S. Pavlovsky, Polymer, 2005, 46, 6682–6694 CrossRef CAS.
- A. Stein, Adv. Mater., 2003, 15, 763–775 CrossRef CAS.
- (a) G.-R. Yi, J. H. Moon and S.-M. Yang, Chem. Mater., 2001, 13, 2613–2618 CrossRef CAS; (b) P. S. Winkel, W. W. Lukens, P. Yang, D. I. Margolese, J. S. Lettow, Y. J. Ying and G. D. Stucky, Chem. Mater., 2000, 12, 686–696 CrossRef.
- A. N. Khramov and M. M. Collinson, Chem. Commun., 2001, 767 RSC.
- H. Zhang and A. I. Cooper, Ind. Eng. Chem. Res., 2005, 44, 8707–8714 CrossRef CAS.
- (a) A. Imhof and D. J. Pine, Nature, 1997, 389, 948–951 CrossRef CAS; (b) T. Sen, G. J. T. Tiddy, J. L. Casci and M. W. Anderson, Microporous Mesoporous Mater., 2005, 78, 255–263 CrossRef CAS; (c) J. L. Blin, R. Bleta, J. Ghanbaja and M. J. Stébé Micropor., Microporous Mesoporous Mater., 2006, 94, 74–80 CrossRef CAS.
- Complementary Medicine Evaluation Committee extracted ratified minutes, sixteenth meeting, 1999.
- (a) T. Reiner, S. Kababya and I. Gotman, J. Mater. Sci.: Mater. Med., 2008, 19, 583–589 CrossRef CAS; (b) J. R. Jones, S. Lin, S. Yue, P. D. Lee, J. V. Hanna, M. E. Smith and R. J. Newport, Proc. Inst. Mech. Eng., Part H, 2010, 224, 1373–1387 CrossRef CAS; (c) A. Hertz and I. J. Bruce, Nanomedicine, 2007, 2, 899–918 CrossRef CAS; (d) J. Moura, L. N. Teixeira, C. Ravagnani, O. Peitl, E. D. Zanotto, M. M. Beloti, H. Panzeri, A. L. Rosa and P. T. De Oliveira, J. Biomed. Mater. Res., Part A, 2007, 82, 545–557 CrossRef; (e) G. L. Yuan, M. Y. Yin, T. T. Jiang, M. Y. Huang and Y. Y. Jiang, J. Mol. Catal. A: Chem., 2000, 159, 45–50 CrossRef CAS; (f) D. M. Liu and I. W. Chen, Acta Mater., 1999, 47, 4535–4544 CrossRef CAS.
- (a) S.-H. Cheng, W.-N. Liao, L.-M. Chen and C.-H. Lee, J. Mater. Chem., 2011, 21, 7130–7137 RSC; (b) A. Tan, S. Simovic, A. K. Davey, T. Rades and C. A. Prestidge, J. Controlled Release, 2009, 134, 62–70 CrossRef CAS; (c) M. Manzano, M. Colilla and M. Vallet-Reg, Expert Opin. Drug Delivery, 2009, 6, 1383–1400 CrossRef CAS; (d) A. Tan, S. Simovic, A. K. Davey, T. Rades, B. J. Boyd and C. A. Prestidge, Mol. Pharmaceutics, 2010, 7, 522–532 CrossRef CAS; (e) S. Simovic, P. Heard, H. Hui, Y. Song, F. Peddie, A. K. Davey, A. Lewis, T. Rades and C. A. Prestidge, Mol. Pharmaceutics, 2010, 6, 861–872 CrossRef.
- (a) S. Das and A. Chaudhury, AAPS PharmSciTech, 2011, 12, 62–76 CrossRef CAS; (b) R. H. Müller, K. Mäder and S. Gohla, Eur. J. Pharm. Biopharm., 2000, 50, 161–177 CrossRef.
- M. A. Schubert and C. C. Müller-Goymann, Eur. J. Pharm. Biopharm., 2003, 55, 125–131 CrossRef CAS.
- T. Helgason, T. S. Awad, K. Kristbergsson, D. J. McClements and J. Weiss, J. Am. Oil Chem. Soc., 2008, 85, 501–511 CrossRef CAS.
- G. Lukowski, J. Kasbohm, P. Pflegel, A. Illing and H. Wulff, Int. J. Pharm., 2000, 196, 201–205 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Experimental details, DLS hydrodynamic diameter measurements, BJH adsorption isotherms, SAXS data of SLN and porous silica. See DOI: 10.1039/c1ra00659b/ |
‡ General procedure of SLN preparation: A cold solution of NHP (10%) in THF was added to a 10 mL dispersion of Tween 20 (1.5%) maintained at 70 °C under magnetic stirring, at a flow rate of 10 mL min−1. The milky suspension spontaneously formed was kept under stirring until the solvent was completely evaporated and the room temperature was reached. General procedure of the material preparation: To a 5 mL dispersion of SLN containing 10% solid lipid and 1.5% Tween 20 was added 150 mg of TMOS at neutral pH and kept at 70 °C for 24 h. Then, the resulting mixture was washed with dichloromethane and ethanol until the solid lipid and the surfactant were completely removed (as verified by FT-IR by following the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band at 1732 cm−1). O band at 1732 cm−1). |
| § Mercury intrusion porosimetry measurements (MIP) were performed on Pascal 140/240 Instrument (Thermo Scientific). |
| This journal is © The Royal Society of Chemistry 2011 |
