A remarkable temperature effect in the desymmetrisation of bridged meso-tricyclic succinic anhydrides with chiral oxazolidin-2-ones†
Narendra R.
Chaubey
and
Sunil K.
Ghosh
*
Bio-Organic Division, Bhabha Atomic Research Centre, Mumbai, India. E-mail: ghsunil@barc.gov.inc; Fax: +91-22-25505151; Tel: +91-22-25595012
First published on 19th August 2011
Abstract
A temperature dependent reversal of diastereoselectivity was observed in the desymmetrisation of bridged meso-tricyclic succinic anhydrides with lithiated chiral oxazolidin-2-ones that appears to be kinetically driven at low temperature while the reversibility of the reaction sets in at higher temperatures and thermodynamics governs the reaction outcome.
Desymmetrisation products of bridged meso-tricyclic succinic anhydrides are well known intermediates in various syntheses.1 A great deal of research has been carried out to achieve high enantiomeric/diastereoisomeric purity by involving an achiral alcohol in combination with a chiral catalyst2–4 or chiral reagents like alcohols5 and amines.6 Chiral oxa-/iso-oxazolines and oxazolidin-2-ones7,8 have recently been introduced for the desymmetrisation of various symmetric anhydrides. The choice of the oxazolidin-2-one class of chiral reagents was made because this fragment is widely used as a powerful auxiliary for controlling diastereoselective reactions9 when attached to a carboxylic acid group and it is easy to remove. While the effect of solvent, concentrations and additives for the desymmetrisation10 of various prochiral anhydrides show a drastic improvement in stereoselectivity and/or even inversion of stereoselectivity, change of temperature is known to have a minimal effect. Herein, we report for the first time a remarkable temperature dependent change of diastereofacial selectivity in the desymmetrisation of bridged meso-tricyclic anhydrides with chiral oxazolidin-2-ones and also established the structural parameters of the oxazolidin-2-ones required for optimum diastereoselectivity.
Lithiated SuperQuat11oxazolidin-2-one 1a (Fig. 1) has been used successfully by us8 for the desymmetrisation of 3-substituted glutaric anhydrides at −78 °C in THF–DMPU. When meso-tricyclic anhydride 2a (Fig. 1) was reacted with Li-1a following the reported8 conditions, diastereoisomeric esters 5a and 6a (5a/6a = 50/50) were isolated in 76% yield after diazomethane esterification (Scheme 1). Similar results were obtained with other tricyclic anhydrides 2b–d leading to methyl esters in good yield but with almost no diastereoselectivity (Fig. 2; 5b/6b = 44/56, 71%; 5c/6c = 52/48, 70%; 5d/6d = 50/50, 75%). Although the results are disappointing, they created a challenge to find a solution to the problem.
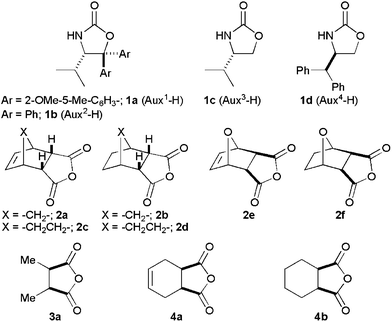 | ||
| Fig. 1 Structures of chiral oxazolidin-2-ones and anhydrides. | ||
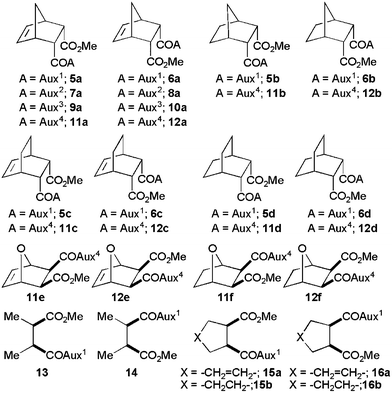 | ||
| Fig. 2 Methyl esters of products from the desymmetrisation of various meso-anhydrides with lithiated oxazolidin-2-ones 1a–d. | ||
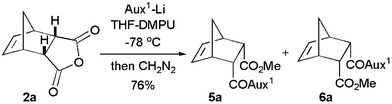 | ||
| Scheme 1 Desymmetrisation of bridged meso-tricyclic anhydride 2a. | ||
Besides other factors,12 temperature plays a significant role in controlling the selectivities of a reaction. Under normal circumstances, the diastereoselectivity of a reaction performed under kinetic control commonly increases with lowering of reaction temperature. An exception to this is reported in certain examples where the selectivity was dramatically increased and/or inverted at higher temperatures.13,14 There are no reports for such instances in anhydride desymmetrisations or reactions involving bridged tricyclic systems.
We therefore, set to study the effect of temperature on the opening of bridged tricyclic anhydride 2a with Li-1a in THF-DMPU (4/1; 0.17M)‡ over a temperature range of −78 °C to 35 °C. No selectivity (Fig. 3) was observed at −78 °C. As the temperature was increased to −28 °C the diastereoselectivity was increased to 36/64 in favor of 6a. Increase in temperature from here decreases the diastereoselectivity until there is nearly no selectivity in the vicinity of 0 °C. With further increase in temperature, a reversal as well as sharp improvement in the diastereoselectivity was seen until about 35 °C§ (5a/6a = 91/9). Thus a simple change in reaction temperature led to products where the lithiated oxazolidin-2-one apparently reacted selectively at every other carbonyl groups of the anhydride 2a.
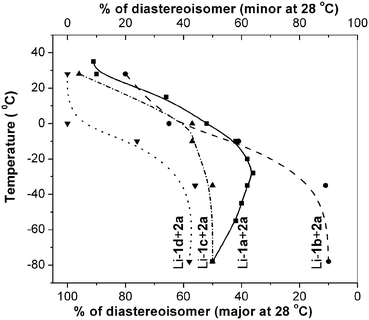 | ||
| Fig. 3 Effect of temperature and oxazolidin-2-one structures on desymmetrisation selectivity of anhydride 2a. | ||
To ascertain the role of the structural feature of oxazolidin-2-ones, if any, anhydride 2a was reacted with lithiated Seebach's,15Evans'16 and Sibi's17oxazolidin-2-ones Li-1b, Li-1c and Li-1d, respectively. At −78 °C, the selectivity was good with 1b providing diastereoisomers 8a and 7a in a ratio of 90/10 and it did not change up to −35 °C (Fig. 3). Between −15 °C and 0 °C, a reversal in selectivity was observed and the diasteroisomer 7a became now major. At 28 °C, a reasonably high selectivity (dr = 20/80) in favor of the diasteroisomer 7a was finally achieved. Between −78 °C and −35 °C, the reaction of 2a with Li-1c or Li-1d (Fig. 3) was unselective and thereafter the selectivity increased slowly followed by a rapid jump above 0 °C. At 28 °C, the diastereoisomer ratio 10a/9a was found to be 96/4. With Li-1d, the reaction became completely diastereoselective providing exclusively the diastereoisomer 11a at 10 °C and above. The SuperQuartoxazolidin-2-ones 1a and 1b showed a clear cut reversal in diastereoselectivity with increasing temperature and an equiselective temperature in the vicinity of 0 °C. The oxazolidin-2-ones 1c and 1d did not show a reversal of selectivity in the temperature range studied but a steady increase in selectivity with the rise in temperature.
To see the generality of this temperature effect, Li-1a was reacted with meso-tricyclic anhydrides 2b–d in THF–DMPU (4/1; 0.17M) at −78 °C and at 28 °C (Table 1). In all cases, the desymmetrisation reactions were unselective at −78 °C but highly selective at 28 °C with excellent yield in all cases (Table 1, entries 2–4). It is interesting to note that meso-, mono- and bi-cyclic anhydrides 3a, 4a and 4b (Fig. 1) showed negligible variation in diastereofacial selectivity (Table 1, entries 5–7) providing the same set of major (13, 15a,b) and minor esters (14, 16a,b) (Fig. 2) at both the temperatures. Thus, the temperature dependent desymmetrisation selectivity does depend more on the anhydride structures than the oxazolidin-2-ones. Only tricyclic meso-anhydrides behaved in this fashion.
| Entry | Anhydride | Products | Diastereoisomer ratioa (% yieldb) | |
|---|---|---|---|---|
| at −78 °C | at 28 °C | |||
| a Determined by 1H NMR of the crude methyl esters. b Combined yield of diastereoisomers. | ||||
| 1 | 2a | 5a, 6a | 50/50 (76) | 90/10 (88) |
| 2 | 2b | 5b, 6b | 44/56 (71) | 93/07 (82) |
| 3 | 2c | 5c, 6c | 52/48 (70) | 92/08 (84) |
| 4 | 2d | 5d, 6d | 50/50 (75) | 96/04 (79) |
| 5 | 3a | 13, 14 | 87/13 (81) | 84/16 (83) |
| 6 | 4a | 15a, 16a | 82/18 (85) | 78/22 (85) |
| 7 | 4b | 15b, 16b | 92/08 (85) | 89/11 (84) |
To probe the generality of the high selectivity of Li-1d, tricyclic anhydrides 2b–f were reacted with it at 28 °C (Table 2). In all cases, the selectivity and yield were excellent. The results are also compared with the selectivity observed at −78 °C. This is also a successful attempt3 to desymmetrise anhydrides 2c and 2d with such high selectivity.
| Entry | Anhydride | Productsa | Diastereoisomer ratiob (% yield) | |
|---|---|---|---|---|
| at −78 °C | at 28 °C | |||
| a Absolute configurations were determined by chemical correlations (see, the Electronic Supplementary Information). b Determined by 1H NMR of the crude methyl esters. c Combined yield of diastereoisomers. | ||||
| 1 | 2a | 11a, 12a | 60/40 | >99/1 (85) |
| 2 | 2b | 11b, 12b | 60/40 | >99/1 (85) |
| 3 | 2c | 11c, 12c | 76/24 | >99/1 (80) |
| 4 | 2d | 11d, 12d | 33/67 | >99/1 (79) |
| 5 | 2e | 11e, 12e | 80/20 | 95/05 (85)c |
| 6 | 2f | 11f, 12f | 48/52 | >99/1 (84) |
To understand the origin of this temperature effect, anhydride 2a was reacted with Li-1a in THF–DMPU at −35 °C or at 0 °C but quenched after raising the reaction temperature to 28 °C. In both cases, the diastereoisomers ratio (5a/6a) found did not match with the values obtained at −35 °C or 0 °C but matched with the values at 28 °C. Furthermore, the same reaction was performed at 28 °C, the temperature was lowered slowly to −35 °C and then the reaction was quenched. In this case, the proportion of methyl esters 5a/6a matched with the values at 28 °C. We have also carried out crossover experiments by reacting anhydride 2a with Li-1a at −35 °C or 28 °C. After 0.5 h, equiv amount of anhydride 2c was added into the reaction mixture and quenched after 10 min at 28 °C. We did not see any transamidation product in both cases and a mixture of products 5a and 6a were formed only from anhydride 2a. We thus speculate that the initial stereoinduction step is the kinetic addition of the Li-1a to one of the prostereogenic carbonyls of the anhydride to give the minor lithium salt 17a and major lithium salt 18a which appeared to be stable at low temperature (Scheme 2). Quenching the reaction at low temperature provided a mixture of methyl ester 5a and 6a at par with kinetic ratio. At higher temperature, the lithium salt 18a transforms into the thermodynamically stable lithium salt 17a by an intramolecular transfer of the oxazolidin-2-one group. Quenching and esterification lead to a mixture of methyl esters 5a and 6a at par with thermodynamic ratio. Thus the kinetically favored diastereoisomer was obtained at low temperature while thermodynamically controlled diastereoisomer was obtained at high temperature quenching. The thermodynamically favored diastereoisomer once formed could not revert to the kinetically favored one even when the temperature was lowered presumably due to conversion of 17a and 18a to carboxylates 19a and 20a, respectively at higher temperature.
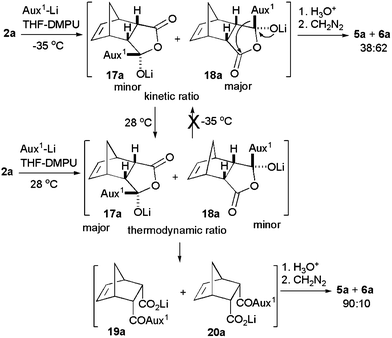 | ||
| Scheme 2 Mechanistic studies. | ||
In conclusion, oxazolidin-2-ones are a class of molecules which in stoichiometric amounts could be efficiently employed for the desymmetrisation of prochiral anhydrides. Bridged tricyclic anhydrides only showed temperature-dependent diastereoselectivity which is specific to their structure. Study of more such related reactions with bridged tricyclic structures are under active investigation.
References
- Selected examples: (a) J. T. Starr, G. Koch and E. M. Carreira, J. Am. Chem. Soc., 2000, 122, 8793 CrossRef CAS; (b) A. Bernardi, D. Arosio, L. Manzoni, F. Micheli, A. Pasquarello and P. Seneci, J. Org. Chem., 2001, 66, 6209 CrossRef CAS; (c) C. Tanyeli and S. Özçubukçu, Tetrahedron: Asymmetry, 2003, 14, 1167 CrossRef CAS; (d) F. Freire, J. D. Fisk, A. J. Peoples, M. Ivancic, I. A. Guzei and S. H. Gellman, J. Am. Chem. Soc., 2008, 130, 7839 CrossRef CAS; (e) N.-C. Kong, Y. Zhang, S. Gao, Y. Lu, Q.-T. Zheng, Q.-Y. Sun, F.-M. Yang, Y.-T. Di and X.-J. Hao, Tetrahedron Lett., 2009, 50, 957 CrossRef CAS; (f) C. L. Campbell, C. Hassler, S. S. Ko, M. E. Voss, M. A. Guaciaro, P. H. Carter and R. J. Cherney, J. Org. Chem., 2009, 74, 6368 CrossRef CAS; (g) T. Koshiba, S. Yokoshima and T. Fukuyama, Org. Lett., 2009, 11, 5354 CrossRef CAS.
- Key papers: (a) M. Shimizu, K. Matsukawa and T. Fujisawa, Bull. Chem. Soc. Jpn., 1993, 66, 2128 CrossRef CAS; (b) D. Seebach, G. Jaeschke and Y. M. Wang, Angew. Chem., Int. Ed. Engl., 1995, 34, 2395 CrossRef CAS; (c) D. Seebach and G. Jaeschke, J. Org. Chem., 1998, 63, 1190 CrossRef; (d) R. A. Aitken and J. Gopal, Tetrahedron: Asymmetry, 1990, 1, 517 CrossRef CAS; (e) C. Bolm, A. Gerlach and C. L. Dinter, Synlett, 1999, 195 CrossRef CAS; (f) C. Bolm, I. Schiffers, C. L. Dinter and A. Gerlach, J. Org. Chem., 2000, 65, 6984 CrossRef CAS; (g) Y. Chen, S. Tian and L. Deng, J. Am. Chem. Soc., 2000, 122, 9542 CrossRef CAS; (h) Y.-M. Song, J. S. Choi, J. W. Yang and H. Han, Tetrahedron Lett., 2004, 45, 3301 CrossRef CAS; (i) T. Honjo, S. sano, M. Shiro and Y. Nagao, Angew. Chem., Int. Ed. Engl., 1995, 34, 2395 CrossRef.
- (a) H. S. Kim, Y.-M. Song, J. S. Choi, J. W. Yang and H. Han, Tetrahedron, 2004, 60, 12051 CrossRef CAS; (b) T. Okamatsu, R. Irie and T. Katsuki, Synlett, 2007, 1569 CAS; (c) R. Manzano, J. M. Andres, M.-D. Muruzabal and R. Pedrosa, J. Org. Chem., 2010, 75, 5417 CrossRef CAS.
- Selected reviews: (a) Y. Chen, P. McDaid and L. Deng, Chem. Rev., 2003, 103, 2965 CrossRef CAS; (b) A. C. Spivey and B. I. Andrews, Angew. Chem., Int. Ed., 2001, 40, 3131 CrossRef CAS; (c) M. C. Willis, J. Chem. Soc., Perkin Trans. 1, 2001, 1765 Search PubMed; (d) I. Atodiresei, I. Schiffers and C. Bolm, Chem. Rev., 2007, 107, 5683 CrossRef CAS.
- Key papers: (a) P. D. Thiesen and C. H. Heathcock, J. Org. Chem., 1993, 58, 142 CrossRef; (b) M. Ohshima and T. Mukaiyama, Chem. Lett., 1987, 377 CrossRef CAS; (c) M. Ohtani, T. Matsuura, F. Watanabe and M. Narisada, J. Org. Chem., 1991, 56, 4120 CrossRef CAS; (d) T. Konoike and Y. Araki, J. Org. Chem., 1994, 59, 7849 CrossRef CAS; (e) H. Imado, T. Ishizuka and T. Kunieda, Tetrahedron Lett., 1995, 36, 931 CrossRef CAS.
- Key papers: (a) M. North and G. Zagotto, Synlett, 1995, 639 CrossRef CAS; (b) I. G. Jones, W. Jones and M. North, Synlett, 1997, 1478 CrossRef CAS; (c) D. E. Hibbs, M. B. Hursthouse, I. G. Jones, W. Jones, K. M. A. Malik and M. North, J. Org. Chem., 1999, 64, 5413 CrossRef CAS; (d) R. S. Ward, A. Pelter, M. I. Edwards and J. Gilmore, Tetrahedron, 1995, 52, 12799 CrossRef.
- (a) S. D. Real, D. R. Kronenthal and H. Y. Wu, Tetrahedron Lett., 1993, 34, 8063 CrossRef CAS; (b) A. C. Evans, D. A. Longbottom, M. Matsuoka, J. E. Davies, R. Turner, V. Franckevicius and S. V. Ley, Org. Biomol. Chem., 2009, 7, 747 RSC; (c) R. Verma, S. Mithran and S. K. Ghosh, J. Chem. Soc., Perkin Trans. 1, 1999, 257 RSC; (d) R. Verma and S. K. Ghosh, J. Chem. Soc., Perkin Trans. 1, 1999, 265 RSC.
- N. Chaubey, S. M. Date and S. K. Ghosh, Tetrahedron: Asymmetry, 2008, 19, 2721 CrossRef CAS.
- D. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835 CrossRef CAS.
- (a) S. H. Oh, H. S. Rho, J. W. Lee, J. E. Lee, S. H. Youk, J. Chin and C. E. Song, Angew. Chem., Int. Ed., 2008, 47, 7872 CrossRef CAS; (b) H. S. Rho, S. H. Oh, J. W. Lee, J. Y. Lee, J. Chin and C. E. Song, Chem. Commun., 2008, 1208 RSC; (c) T. Ivsic and Z. Hamersak, Tetrahedron: Asymmetry, 2009, 20, 1095 CrossRef CAS; (d) N. Hashimoto, S. Kawamura, T. Ishizuka and T. Kunieda, Tetrahedron Lett., 1996, 37, 9237 CrossRef CAS.
- S. G. Davies and H. J. Sanganee, Tetrahedron: Asymmetry, 1995, 6, 671 CrossRef CAS.
- Selected reviews: (a) M. Bartok, Chem. Rev., 2010, 110, 1663 CrossRef CAS; (b) T. Tanaka and M. Hayashi, Synthesis, 2008, 3361 CAS.
- Selected reviews: (a) H. Buschmann, H.-D. Scharf, N. Hoffmann and P. Esser, Angew. Chem., Int. Ed. Engl., 1991, 30, 477 CrossRef; (b) G. Cainelli, D. Giacomini and P. Galletti, Chem. Commun., 1999, 567 RSC.
- Selected examples: (a) T. Gobel and K. B. Sharpless, Angew. Chem., Int. Ed. Engl., 1993, 32, 1329 CrossRef; (b) G. Pandey, K. N. Tiwari and V. G. Puranik, Org. Lett., 2008, 10, 3611 CrossRef CAS; (c) B. M. Trost, A. Fettes and T. Shireman, J. Am. Chem. Soc., 2004, 126, 2660 CrossRef CAS; (d) J. Mowat, B. Kang, B. Fonovic, T. Dudding and R. Britton, Org. Lett., 2009, 11, 2057 CrossRef CAS; (e) X.-W. Sun, M.-H. Xu and G.-Q. Lin, Org. Lett., 2006, 8, 4979 CrossRef CAS; (f) Z.-Z. Huang, Y.-B. Kang, J. Zhou, M.-C. Ye and Y. Tang, Org. Lett., 2004, 6, 1677 CrossRef CAS; (g) C. Rondot and J. Zhu, Org. Lett., 2005, 7, 1641 CrossRef CAS; (h) M. P. Sibi, U. Gorikunti and M. Liu, Tetrahedron, 2002, 58, 8357 CrossRef CAS; (i) G. Cainelli, D. Giacomini and P. Galletti, Eur. J. Org. Chem., 1999, 61 CrossRef CAS; (j) G. Cainelli, D. Giacomini, P. Galletti and A. Quintavalla, Eur. J. Org. Chem., 2003, 1993 CrossRef CAS; (k) A. Gromov, V. Enev and J. Mulzer, Tetrahedron Lett., 2009, 50, 5283 CrossRef CAS.
- T. Hintermann and D. Seebach, Helv. Chim. Acta, 1998, 81, 2093 CrossRef CAS.
- D. A. Evans and A.E. Weber, J. Am. Chem. Soc., 1986, 108, 6757 CrossRef CAS.
- M. P. Sibi, Aldrichimica Acta, 1999, 32, 93 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Typical experimental procedure, full characterization data, copies of 1H and 13C NMR. See DOI: 10.1039/c1ra00483b |
| ‡ It was established that this solvent combinations and reaction concentration were essential for optimal selectivity and yield. |
| § Above this temperature, the product yield dropped sharply with formation of unidentified by-products. |
| This journal is © The Royal Society of Chemistry 2011 |
