Pd-based membrane reactors for one-stage process of water gas shift
G.
Barbieri
*a,
A.
Brunetti
a,
A.
Caravella
a and
E.
Drioli
ab
aNational Research Council – Institute for Membrane Technology (ITM–CNR), c/o The University of Calabria, cubo 17C, Via Pietro BUCCI, 87036 Rende CS, Italy. Web: www.itm.cnr.itE-mail: g.barbieri@itm.cnr.it; Fax: +39 0984 402103; Tel: +39 0984 492029
bThe University of Calabria – Department of Chemical Engineering and Materials, cubo 44A, Via Pietro BUCCI, 87036 Rende CS, Italy
First published on 23rd August 2011
Abstract
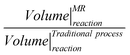
The water gas shift (WGS) reaction is the upgrading stage in the cycles of hydrogen production by, for example, steam reforming of light hydrocarbons from fossil or renewable sources. This is a thermodynamics limited reaction and CO conversion is furthermore depleted by the presence of products, such as hydrogen as often/always happens in industrial applications. WGS industrial processes consist of two reactors in series: the first one operates at high temperature (300–400 °C), exploiting the advantages offered by a fast kinetics; the second one works in the low temperature range (200–300 °C) to the benefit of the higher thermodynamic conversion. This work proposes the use of one Pd-based membrane reactor (MR) operating at the same temperature range as the high temperature WGS reactor as a suitable alternative to the whole traditional reactor (TR) process. The hydrogen permeation allows the increase of the equilibrium conversion close to the total value and thus to operate in the higher temperature range exploiting the greater kinetics offered by Fe–Cr based catalysts. The values of gas hourly space velocity (GHSV), temperature, H2O/CO feed molar ratio, feed composition, etc. used in the simulations are those typical of an industrial application of a WGS upgrading stage. A reference value of 15 bar of feed pressure was assumed since this is the strength limit of the self supported Pd–Ag membrane considered in the simulations. However, a feed pressure of 30 bar was also considered as that used in industrial processes. The pressure proves to be one of the most interesting variables of MR processing. The proposed analysis demonstrates how only one stage based on a Pd-alloy MR can replace the two reactors of the traditional process, which also gives better performance for, e.g., CO conversion, pure hydrogen recovered on the permeate stream, etc. An intensified process with a smaller reaction volume, higher conversion and GHSV, etc. is the outcome.
Introduction
The Hydrogen Posture Plan, published by EERE in February 2004, envisaged a complete transition to a hydrogen economy by 2030–2040.1 At present, the global hydrogen production mainly relies on processes that extract hydrogen from fossil fuel feedstock. 96% hydrogen is produced directly from fossil fuels and about 4% is produced indirectly by using electricity generated through fossil fuels.2 The main challenge of the forthcoming years is the increasing of the percentage of H2 produced by biomass or by bioprocesses. In the meantime, new technologies, allowing a better exploitation of fossil fuels (e.g. light hydrocarbons) and also of bio-sources, constitutes an important step toward the development of intensified processes.Traditionally, syngas mixtures containing mostly hydrogen and carbon monoxide can be generated in industrial processes at elevated temperatures by steam reforming or partial oxidation of light hydrocarbons or bio-fuels or gasification of coal and biomass and, then, fed to an upgrading stage for reducing the CO content and producing more H2. This upgrading stage consists of the water gas shift (WGS) reaction which is exothermic and characterized by no variation of the mole number:
| CO + H2O = CO2 + H2 ΔH0298 = −41 kJ mol−1 | (21) |
Therefore, CO conversion is thermodynamically favoured by low temperatures but, on the contrary, its kinetics is promoted by a high temperature.
Fe2O3–Cr2O33,4,5 is the most used catalyst to convert CO into the high temperature (300–450 °C) range, where conversion is strongly limited by thermodynamics. These catalysts show high kinetic activity when operated at a temperature higher than 300 °C. A further CO conversion is achieved in a low temperature stage using CuO–ZnO3,5,6 or CuO–CeO2 based catalysts. These catalysts undergo sintering at a higher temperature and present a lower reaction rate with respect to Fe2O3–Cr2O3 catalysts; therefore, usually the typical GHSV (gas hourly space velocity) of the second stage is ca. tenfold lower than the high temperature reactor. For these reasons, the WGS conventional process (Fig. 1b, “traditional process”) generally consists of:
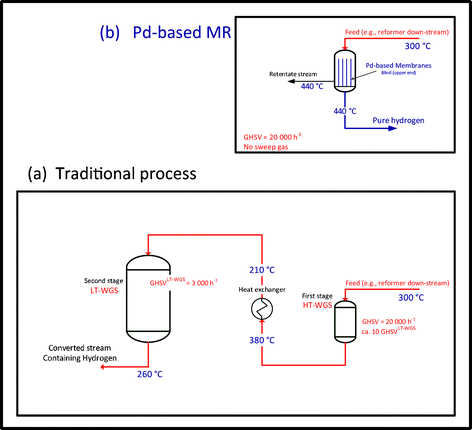 | ||
| Fig. 1 Schemes of “Pd-based MR” and “traditional process” for WGS reaction. The temperature values reported are indicative of a typical operation. | ||
1) HT-WGS, a first reactor operating at high temperatures (320–450 °C) and GHSV (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1)
000 h−1)
2) A heat exchanger for cooling the stream from 400–450 °C to ∼200 °C
3) LT-WGS, a second reactor operating at low temperatures (210–300 °C) and GHSV (2000 h−1).
Both HT- and LT-WGS reactors operate industrially in (or close to) adiabatic conditions. Industrially, steam is added to the syngas feed stream in order to shift the thermodynamic equilibrium conversion and increase the forward reaction rate, thus the hydrogen yield is enhanced too. Therefore, the typical H2O/CO feed molar ratio ranges from 2 to 5.5
The H2 rich stream coming out from the TR-process is, usually, directed to a pressure swing adsorption (PSA) unit for H2 separation from the other gases and in particular CO, which poisons the catalysts of industrial processes. In addition, new utilization of H2, e.g., in fuel cells for mobile and stationary power sources, requires a CO concentration lower than 10 ppm7 in the anode inlet feed to avoid catalyst poisoning with consequent drop in the cell efficiency. Hence, a further purification of the produced H2 from carbon-containing sources is required and it must be very efficient to reduce the CO level to cell requirements. Fig. 1b does not show these additional units required for the separation and purification of hydrogen.
The improvement of the WGS technology for hydrogen production is growing due to the specific interest of the energy industry. For this reason, membrane reactors (MRs) (Fig. 1a) able to couple the WGS reaction and hydrogen separation using a selective membrane are currently studied both experimentally and theoretically at low8,9,10,11,12 and high13,14 temperatures, respectively. The latter are mainly focused on the study of the catalyst performance and/or on the experimental analysis with a home-made membrane.
The Pd-alloy membranes are the most used for selective hydrogen removal in applications such as dehydrogenation reactions15,16,17,18,19 because of their infinite H2 selectivity. Hydrogen removal gives several advantages with respect to traditional operations:
• depleting the reverse reaction rate due to the lower H2 concentration
• increasing the residence time of reactants
• exceeding the thermodynamic equilibrium of a traditional reactor.
• pressure was demonstrated20 to have a positive effect on conversion in an MR for reactions characterised by an increase of the mole number such as, e.g., methane steam reforming as well as WGS (having no variation in the number of moles) even though, in a TR, it depletes or does not affect the conversion of methane steam reforming and WGS, respectively.
Currently, self-supported Pd–Ag membranes are produced at commercial level.21,22 These membranes were used to investigate experimentally the WGS reaction8,19 at lab scale. They exhibited a high durability and stability in performance for more than two years, undergoing several start-ups and shutdowns operating at 330 °C and up to 10 bar. Tokyo Gas has been developing a highly efficient and compact hydrogen separation reformer with less CO2 emissions. It produces 40 Nm3/h of pure hydrogen from town gas using a hydrogen separation membrane.23
This work presents an analysis of the performance of a Pd–Ag MR for the WGS reaction, the upgrading stage in the hydrogen production process, highlighting the peculiar advantages offered by this new reactor model. In particular, CO conversion, hydrogen production as well as reaction volume and catalyst amount required were analysed as a function of feed pressure and H2O/CO feed molar ratio at the typical temperatures of WGS industrial processes and also considering the most used and widely diffused catalysts.
MR was analysed only at high temperature, whereas the traditional process needs both HT- and LT-WGS, to achieve a performance close to that of MR. The MR overcomes the limitation imposed by the thermodynamics to the HT-WGS stage due to hydrogen removal and, hence, the fast kinetics characterizing the Fr–Cr based catalysts can be successful exploited.
Industrially, the H2O/CO feed molar ratio ranges up to 5 depending on the type of stream to be treated and on the target required,5 it is significantly higher than the stoichiometric value of 1. A higher feed molar ratio increases the CO conversion, which is strongly limited by the thermodynamics as well as by the reaction rate. In addition, the syngas stream is often produced by steam reforming or partial oxidation of light hydrocarbons or coal gasification. In all these processes an excess of water is used to improve the conversion and/or to overcome/reduce the coke formation which deactivates the catalyst. Therefore, a H2O/CO feed molar ratio of 3 was used even if very good results were obtained for the stoichiometric feed molar ratio in the MR.
Finally, the present analysis shows a reduction of two reactions and one purification stage in only one unit, the MR. This leads to a simpler and more sustainable process and implies important reductions in the footprint area occupied by the upgrading step. A relevant reduction of the costs related to the equipment, the catalyst, etc. is also expected.
Methods
The mathematical model used in this work has already been published and validated with experimental results in an our previous paper24 and it is reported in the appendix. It was written in terms of dimensionless variables for describing the steady-state behaviour of a non-isothermal Pd-based membrane reactor. The model considered the following hypotheses:• plug flow in the retentate and permeate streams
• constant pressure on the permeate side
• the pressure drop on retentate side was taken into account by Ergun’s law.
A Pd–Ag self-supported commercial membrane (60 micrometres thick, Johnson Matthey®), showing infinite hydrogen selectivity, was considered in the simulations. This type of membrane offers high mechanical resistance with the maximum operating pressure being 16 bar as indicated by the producer.25 Furthermore, it was already tested up to 10 bar (feed pressure) in reaction experiments.8 Therefore, it proved to be suitable for its use in MR, where the role of the driving force is exclusively assigned to the feed pressure, as in this work. This membrane was considered as working in a temperature range in which the diffusion in the metal bulk is the rate determining step; therefore, Sieverts’s law (eqn (1)) was used for the mathematical description of H2 permeating flux. The parameters (Permeance0 = 16.2 mmol m−2 s−1 Pa−0.5; E/R = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 K) measured by Barbieri et al.26 on the same membrane was used in the simulations of this work.
098 K) measured by Barbieri et al.26 on the same membrane was used in the simulations of this work.
 | (1) |
The energy balance considers the MR adiabatic with the ambient and includes energy transfer through the membrane between lumen and shell sides.
The TRs were simulated with the same code, setting to zero both the H2 permeating term and overall heat transfer coefficient between the reaction and permeation sides.
In the simulations, an MR consisting of two concentric tubes was considered (Fig. 2). The outer tube is the stainless steel shell; the inner tube is the self-supported Pd-alloy membrane. The catalyst is packed in the annulus between the two tubes. Table 1 reports the geometric characteristics of the MR and TRs used in the simulation. The greater shell diameter of the MR with respect to the HT-WGS reactor is due to the membrane presence. The lower GHSV of the LT-WGS imposes a higher reaction volume with respect to the HT-WGS step. Both a greater reactor diameter and length are suitable and equivalent solutions for the present modelling analysis; thus, the former was chosen in this work for having the same pressure drop.
 | ||
| Fig. 2 MR scheme considered in the simulations | ||
A typical composition of a syngas stream (Table 2) coming out from a reformer was considered as reference for the feed stream. Of particular industrial interest, this mixture contains the reactants (CO and H2O), products, specifically and quantitatively hydrogen, which is the permeable species, as well as inert gas (N2). Table 2 reports the operating conditions considered in the simulations as a reference study, which are very close to the ones currently used at industrial level for the traditional process. In the MR the permeation driving force was generated only by feed pressure. No sweep gas was used. Feed pressure was kept lower at 15 bar, since this is the limit of the self supported Pd–Ag membrane considered in the present work. MR was also simulated at 30 bar, a value closer to those typically used in industrial processes (20–30 bar). The higher pressure used in industrial processes for volume reduction does not affect the thermodynamics of this process. It has little effect on the kinetics as in the MR. The same pressure was considered in the simulation of the traditional process and the same reactor length was chosen for the MR, HT-WGS and LT-WGS reactors which have the same pressure drop. Also an H2O/CO feed molar ratio of three was considered in this analysis.
| Pd–Ag MR | Traditional process (HT-WGS, 1st stage) | |
|---|---|---|
| Inlet Temperature | 300 °C | |
| Feed pressure | 15 bar | |
| Feed mixture composition | CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 52 N2 = 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.5 20.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 % molar (dry basis) 8.5 % molar (dry basis) |
|
| H2O/CO feed molar ratio | 1 | |
| GHSV | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 000 h−1 |
|
The second, LT-WGS, stage of the traditional process was fed with the down stream of the HT-WGS (first stage of the Traditional process) and operates at a GHSV of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 and 210 °C.
000 h−1 and 210 °C.
In addition, temperature, GHSV, feed pressure were ranged from 300 to 400 °C, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 40
000 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, 5–15 bar, respectively, out of the reference values reported in Table 2, in order to investigate another important aspect of the MR.
000 h−1, 5–15 bar, respectively, out of the reference values reported in Table 2, in order to investigate another important aspect of the MR.
The CO conversion (eqn (2)) is one of the most important variables to evaluate and compare the traditional process and MR performance. The MR performance was also evaluated by means of Hydrogen Recovery (eqn (3)),27 a lumped parameter, which allows the fraction of hydrogen permeated through the membrane to be estimated, with respect to the whole, present (feed or produced by reaction) in the system.
 | (2) |
 | (3) |
 | (4) |
The upper limit of a chemical reaction is given by the thermodynamic equilibrium conversion. It is well known for a TR, here is indicated as TREC, TR equilibrium conversion. The equilibrium of an MR, introduced in the last few years,28 requires that the permeation equilibrium has to be reached in addition to the reaction equilibrium, typical of a TR. Therefore, the MR equilibrium conversion (MREC) is a function of the thermodynamic variables (e.g. T, P) and the initial compositions on both sides of the Pd-alloy membrane. It corresponds to the maximum conversion achievable in the MR and depends on the extractive capacity of the system. MREC is always higher than TREC thus also the actual conversion of an MR is expected higher than that of a TR.
The stage-cut (eqn (4)), a well-known parameter in the membrane gas separation field, gives good information about the subdivision of the two outlet streams. For the Pd-based membrane operation, the highest stage-cut value is limited to the sum of the hydrogen fed and produced by the reaction. In the present case, the amount of hydrogen producible (the maximum value) is equal to the CO, which is the limiting reactant. For instance, this maximum stage-cut is 59%, since the hydrogen and CO molar fractions (on wet basis for H2O/CO feed molar ratio of one) are 0.43 and 0.16, respectively. For a higher H2O/CO feed molar ratio this value is lower, i.e., the presence of more water reduces the molar fraction of the other species.
Results and Discussion
As a case study to compare the performances of the MR and traditional process, the simulations were carried out considering the operating conditions reported in Table 2. The inlet temperature and GHSV selected are typical values used in industrial applications.5 An equimolecular feed molar ratio was chosen as a reference, in order to show the advantage offered by the MR; later, a second case study will present the results obtained for a feed molar ratio of three, which is a value closer to those of industrial applications.Fig. 3 compares CO conversion as a function of the temperature obtained for the MR and the traditional process operating at the same inlet conditions, i.e., same GHSV and temperature for the MR and the traditional process (first stage). The MR is operated only in one stage; whereas, the traditional process consists of three steps (Fig. 1). In fact, the down-stream in the first reactor of the traditional process is cooled (at 210 °C) before its feeding to the second stage (LT-WGS), which works at lower GHSV (3000 h−1). Fig. 3 shows the adiabatic reaction paths (solid lines), highlighting the exit conversion by means of symbols, of the MR and traditional process (both stage) and the equilibrium conversion of a TR (TREC) and of an MR (MREC), the latter calculated at 15 bar, the same reaction pressure considered in the simulations. The CO conversion achieved by MR is around 10% higher than the overall one of the traditional process; it also exceeds significantly (ca. 25–30%) the TREC. The hydrogen removal from the reaction side due to the permeation shifts the reaction towards further conversion. This effect is well operated in this MR since a reaction pressure of 15 bar promotes the hydrogen permeation well. This gain is clearer considering that the MR conversion is ca. 33% higher than that achieved by the first stage of the traditional process (HT-WGS).
 | ||
| Fig. 3 CO conversion as a function of temperature for MR and Traditional process. Operating conditions as in Table 2. | ||
The adiabatic reaction paths of the MR and traditional process begin from the same starting point at 300 °C and zero conversion. Whereas, the temperature of the traditional process (first stage) reaches ca. 370–380 °C and cannot go over the equilibrium limitation, that of the MR achieves a higher value (ca. 450 °C) to which corresponds a higher conversion. The MR temperature profile (Fig. 4) is lower on the right with respect to that of the traditional process. At the same conversion, corresponds the same amount of heat generated by the reaction. In the MR, the same conversion is achieved in a shorter reactor length and hence the energy generated (the same value in both cases) has to heat a smaller reaction volume with respect to the HT-WGS reactor. The MR thermal capacity of the reacting system, included between the reactor entrance and the reactor length, where the same conversion achieved is smaller, since the density of the gaseous reactant stream is the same in both cases. Fig. 4 shows a comparison of the CO conversion and temperature profiles of both the MR and the traditional process, the abscissa being the ratio of the catalyst weight with respect to that of the MR, i.e., the reverse of the GHSV ratio. CO conversion in the MR is always higher than that of the traditional process; the latter also shows a slope discontinuity in correspondence to the cooling (for an abscissa value of 1) between the two HT-WGS and LT-WGS reactors. The final conversion of the traditional process is reached only considering a catalyst amount ca. 10 times bigger than that packed in the MR. The higher temperature of the MR allows the utilization of Fe2O3–Cr2O3 catalysts for the whole reaction, owing to the reaction shifting following the permeation. On the contrary, the traditional process after the intermediate mandatory cooling has use CuO–ZnO- or CuO–CeO2-based catalysts, which are characterized by much slower (ca. tenfold) kinetics.
 | ||
| Fig. 4 CO conversion and temperature profiles of MR and the traditional process versus the ratio between the catalyst weight of the considered process and that of the MR. Operating conditions as in Table 2. | ||
Most of the hydrogen produced is recovered as pure gas in the permeate stream (Fig. 5) of the Pd–Ag MR and, owing to its quality, it can be directly fed to a PEM fuel cells. In addition, the retentate is compressed and concentrated in CO2 (65% molar) and, thus, CO2 can be more easily captured, resulting in a relevant/important reduction in the successive separations. On the contrary, the H2 exiting from the traditional process (at more or less 60% molar) is still mixed with the other gases (Fig. 5) and, in particular, with ca. 5.5% CO that must be drastically reduced and, thus, requires a further separation/purification stage before further use. H2O/CO feed molar ratios significantly greater than the stoichiometric value are usually operated at industrial level, as will be discussed later, in order to improve the performance, i.e., CO conversion and hydrogen production of the upgrading stage. Moreover, the CO2 concentration of the residual stream can be close to 70% only if all the H2 present in the stream is separated. Its concentration does not exceed 60% considering the actual separation efficiency of industrial PSA, where the H2 recovery does not exceed 80–90%.29,30
 | ||
| Fig. 5 Outlet stream composition of the MR and the traditional process. Operating conditions as in Table 2. | ||
The dependence of the MR performance on the temperature and pressure was investigated as shown in Fig. 6 and compared with that achieved by the HT-WGS (the first stage of traditional process), in the same operating conditions, in order to understand the differences between the two reacting systems. Any point of the four curves represents the outlet conversion and temperature of the MR. The full symbol shown is the same as reported in Fig. 4. In the whole temperature range investigation, CO conversion achieved in MR is significantly higher than that achieved in HT-WGS and exceeds the TREC for temperatures higher than 370 °C and pressure exceeding 5 bar. CO conversion curves, simulated for the three feed pressures, initially follow an increasing trend with the temperature reaching up to a maximum, followed by a slight decrease.
 | ||
| Fig. 6 Outlet conversion versus outlet temperature of MR and the traditional process for three feed pressures. | ||
This trend is explained considering that both the reaction kinetics and the H2 permeation are favoured by high temperature since they follow Arrhenius’s law. Thus, CO conversion increases with temperature and then reduces once the thermodynamics constraint becomes relevant. The decreasing behaviour is less evident at the high feed pressure, the H2 permeation being more and more promoted as well as the MREC having a lower slope. The highest conversion (ca. 75%), in fact, is achieved at the highest considered feed pressure (15 bar).
The H2 fraction collected in the permeate, with respect to that totally fed/produced into the MR, is quantified in terms of Recovery index (eqn (3)). Its value depends on the permeating capacity of the system; therefore the higher the temperature and feed pressure the higher the recovery index (Fig. 7). At 15 bar and ca. 450 °C, around 90% of the H2 fed or produced by the reaction is recovered as pure H2 in the permeate of the MR.
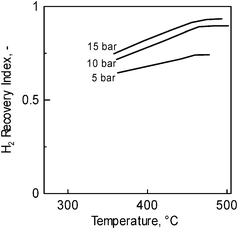 | ||
| Fig. 7 Hydrogen recovery index as a function of temperature for three feed pressures. Operating conditions as in Table 2. | ||
The GHSV is a variable generally used to indicate the reverse of the residence time of reactants over a catalytic bed. A low GHSV indicates a high residence time and, thus, favours the conversion, whereas the contrary applies for a high GHSV. However, a high GHSV is highly desirable since this means a low catalyst amount converts a high feed flow rate and, in addition, low reactor volume is required.
Fig. 8 shows the MR CO conversion and the corresponding H2 recovery index as a function of GHSV for three feed pressures. CO conversion of the HT-WGS is also reported for comparison (dashed line). An increase of GHSV corresponds to a CO conversion decrease. This trend is greatly emphasized with the much higher feed pressure of the MR, since the conversion at the lowest GHSV considered is significantly higher and thus there is much more room for its reduction. However, in all cases, the MR CO conversion is higher than the HT-WGS one (5 times higher @ 15 bar and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1) and also exceeds the TREC at a lower GHSV and above 10 bar. As a consequence, the hydrogen recovery has the same decreasing dependence on the GHSV. A high CO conversion means, in fact, a high H2 production or, rather, a high H2 partial pressure on the reaction side. This is traduced into a high permeation driving force and, thus more H2 recovered in the permeate. In particular, H2 recovery is always higher at 20
000 h−1) and also exceeds the TREC at a lower GHSV and above 10 bar. As a consequence, the hydrogen recovery has the same decreasing dependence on the GHSV. A high CO conversion means, in fact, a high H2 production or, rather, a high H2 partial pressure on the reaction side. This is traduced into a high permeation driving force and, thus more H2 recovered in the permeate. In particular, H2 recovery is always higher at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 and 15 bar, reaching a value of 92%.
000 h−1 and 15 bar, reaching a value of 92%.
 | ||
| Fig. 8 CO conversion in the MR and in the first stage of the traditional process (HT-WGS) and hydrogen recovery index as a function of GHSV. Operating conditions as in Table 2. Three feed pressures were investigated for the MR. | ||
The MR was also simulated for a 30 bar feed pressure, because this value has a higher industrial interest, even if no such resistant self-supported membrane is available on the market yet. The results are very interesting in particular at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, the highest GHSV considered, where the conversion of 80% is much higher (ca. 4 times) than the one achieved at 15 bar by the same MR. The higher pressure significantly favours hydrogen permeation, in fact, the stage-cut is 55% instead of 30% at 15 bar.
000 h−1, the highest GHSV considered, where the conversion of 80% is much higher (ca. 4 times) than the one achieved at 15 bar by the same MR. The higher pressure significantly favours hydrogen permeation, in fact, the stage-cut is 55% instead of 30% at 15 bar.
In this work the MR is proposed as a suitable alternative to the traditional process currently used in the industrial upgrading stage, it is interesting to compare the gain offered by this innovative technology in terms of size reduction. Fig. 9 shows the comparison between the reaction volume required by an MR with respect to that required for the traditional process, for achieving the same conversion, as a function of the feed pressure for inlet temperatures of 300 and 325 °C. The evaluation was made, firstly, calculating CO conversion achieved in the traditional process (with a defined reaction volume for each HT- and LT-WGS reactors) for its suitable operating conditions; then, evaluating the reaction volume required by the MR to obtain the same CO conversion. It depends on the LT-WGS operating at much lower GHSV (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1) owing to the low kinetics of the CuO–ZnO catalyst and temperature of 220–300 °C requires a significantly higher volume. This means a much larger amount of catalyst used to convert a relatively small feed flow rate and it counts for a lot in the determination of the reaction volume of the whole traditional process. As expected, the reaction volume required by MR results is always lower than that of the whole traditional process and it is much lower with a much higher feed pressure. For an inlet temperature of 300 °C, at 5 bar, the MR reaction volume is around 90% of the traditional process, owing to a limited H2 permeation, which causes the MR to work only slightly better than the TR (Fig. 9). This value drastically reduces at higher feed pressure, becoming ca. 13% at 15 bar. Furthermore, at a higher temperature than 325 °C, it is still reduced passing from 55% at 5 bar to ca. 10% at 15 bar. A high feed pressure and a high temperature, imply that more H2 permeates through the membrane and requires less catalyst to achieve a set conversion. A higher pressure (30 bar) produces a further reduction of the reaction volume of the MR, as shown for a GHSV of 40
000 h−1) owing to the low kinetics of the CuO–ZnO catalyst and temperature of 220–300 °C requires a significantly higher volume. This means a much larger amount of catalyst used to convert a relatively small feed flow rate and it counts for a lot in the determination of the reaction volume of the whole traditional process. As expected, the reaction volume required by MR results is always lower than that of the whole traditional process and it is much lower with a much higher feed pressure. For an inlet temperature of 300 °C, at 5 bar, the MR reaction volume is around 90% of the traditional process, owing to a limited H2 permeation, which causes the MR to work only slightly better than the TR (Fig. 9). This value drastically reduces at higher feed pressure, becoming ca. 13% at 15 bar. Furthermore, at a higher temperature than 325 °C, it is still reduced passing from 55% at 5 bar to ca. 10% at 15 bar. A high feed pressure and a high temperature, imply that more H2 permeates through the membrane and requires less catalyst to achieve a set conversion. A higher pressure (30 bar) produces a further reduction of the reaction volume of the MR, as shown for a GHSV of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1.
000 h−1.
 | ||
| Fig. 9 Ratio between the MR volume and volume of the traditional process as a function of feed pressure for inlet temperatures of 300 and 325 °C. Other operating conditions as in Table 2. | ||
Fig. 10 shows the same analysis already proposed in Fig. 3 but for an H2O/CO feed molar ratio equal to three instead of one. The higher value of feed molar ratio gives a higher equilibrium conversion for MR and TR, MREC and TREC, respectively. As a consequence, the actual conversion of the MR and traditional process are higher too with respect to those of Fig. 3. As expected, an improvement in CO conversion of both the traditional process and the MR was obtained for this more interesting case with respect to the ones reached feeding the reactants in equimolecular ratio. However, also in this case, CO conversion of ca. 95% obtained with the Pd-based MR is very high and much higher than that of the traditional process (ca. 86%) and it exceeds the TREC by a very large amount. The reaction paths of the traditional process and MR diverge for the same reason as in Fig. 3, but in this case are close, owing to the greater heat capacity of the reactant streams containing more water (higher feed molar ratio). The water also affects the outlet temperature that is a little lower than the previous case.
 | ||
| Fig. 10 CO conversion as a function of temperature for the traditional process and MR. Operating conditions as in Table 2 except for the H2O/CO feed molar ratio (m)=3. | ||
The higher feed molar ratio of the three values considered in this second case study, even though the CO concentration reduces (Fig. 11) in the traditional process with respect to the other case study, is not enough to meet the limit tolerated by the PEM fuel cells of 10–20 ppm. Therefore, it requires treatment in a further purification step. As widely described before, this is not required by the MR, the permeate stream being completely pure.
 | ||
| Fig. 11 Outlet stream compositions of the traditional processes and MR. Operating conditions as in Table 2 except for the H2O/CO feed molar ratio =3. | ||
The gain offered by the MR in terms of reduction in the reaction volume required to obtain a set CO conversion was quantified considering three different values of feed molar ratio (Table 3). In all cases, the MR volume is significantly lower than that of the traditional process and, as also previously mentioned, this is mainly due to the low temperature stage characterized by the large reaction volume due to the slow kinetics being no longer required. However, the MR proves much more convenient in relation to the closeness of the feed molar ratio to the stoichiometric one. The reaction volume of the MR for the stoichiometric feed molar ratio of 1 is only 13% of that of the traditional process and increases to 33% when a feed molar ratio is five times higher. The excess of water diluting the feed stream is responsible for the increased reaction volumes of both the traditional process and MR.
| H2O/CO feed molar ratio, - |
|
|---|---|
| 1 | 13 |
| 3 | 21 |
| 5 | 33 |
The comparison reported in Table 4 by means of some process variables, shows for both values of H2O/CO feed molar ratio, the better performance of the MR with respect to the traditional process. CO conversion, exiting temperature, hydrogen recovery and volume ratio have already been discussed in previous figures and are reported for completeness. Some comments have to be addressed regarding the other parameters. The CO molar fraction shows an apparent contradiction, since it is higher for the MR with respect to the traditional process. The hydrogen permeation depletes the hydrogen molar fraction and this increases the molar fraction of the other non-permeable species, i.e., CO, CO2 and H2O. The higher CO molar fraction is coupled to a higher CO conversion; in fact, the outlet flow rate of CO is lower for MR and, specifically, it is 82% of that of the TR for the higher feed molar ratio. The higher CO2 molar fraction is an innovative advantage for CO2 separation/sequestration; in fact, a CO2 concentrated stream requires a minor effort in the next stages of the overall process. Finally, the MR system is not optimized and this means hydrogen is significantly present on the retentate side. This hydrogen can be recovered in the same way as that of the traditional process considering the lower load to the downstream separation units. The very high value of stage-cut (ca. 50%) states that the permeate flow rate containing only hydrogen is about the same as the flow rate of the retentate stream including all the species. Thus, the load of the down-stream post treatments is more or less proportionally reduced to this value.
| MR | Traditional process | MR | Traditional process | |
|---|---|---|---|---|
| H2O/CO feed molar ratio | 1 | 3 | ||
| a a cooling from 365 and 379 °C for feed molar ratio of 1 and 3, respectively to 210 °C between the HT- and LT-WGS included | ||||
| CO conversion (%) | 73.5 | 51.6 | 94.8 | 87.2 |
| H2 recovery index (%) | 91.5 | 0 | 9.7 | 0 |
| H2 molar fraction on the exit (feed side), - | 0.096 | 0.545 | 0.085 | 0.429 |
| CO2 molar fraction on the exit (feed side), - | 0.579 | 0.275 | 0.40 | 0.183 |
| CO molar fraction on the exit (feed side), - | 0.092 | 0.055 | 0.011 | 0.053 |
 , molar (%) , molar (%) |
95.5 | 100 | 82 | 100 |
 (%) (%) |
∼52 | N/A | ∼43 | N/A |
 (%) (%) |
13 | 100 | 21 | 100 |
| Temperature (outlet), °C | 456 | 279a | 440 | 264a |
Fig. 1 shows the two proposed processes and includes information elaborating on results and discussion section. In fact, this figure was elaborated to give a graphical comparison since the various elements (reactors) are in due proportion, i.e., the MR and HT-WGS have the same size whereas LT-WGS is close to ten times higher. The further improvement given by the highest pressure of 30 bar was not included in this figure.
Conclusions
The performances of the MR (only one reaction stage) and that of the whole traditional process consisting of two (high and low temperature) reactors were compared as a function of the main variables, such as temperature, feed pressure, H2O/CO feed molar ratio, and space velocity. MR always achieved a very high CO conversion of 80% and 95% for H2O/CO feed molar ratios of 1 and 3, respectively. These conversion values are always higher than those of the whole traditional process and significantly exceed the TR equilibrium conversion (TREC). Therefore, this comparison leads us to conclude, as the first important result, that only one MR can replace the two reactors of the traditional process. The MR operating at the higher temperature regime allows the kinetically faster Fe–Cr-based catalyst to be used, implying, as a consequence, a very large (ca. tenfold) reduction of the total catalyst required by the traditional process for a feed molar ratio of 1. In fact, the presence of the low temperature reactor required for the traditional process, where the Cu-Zn catalyst requires a space velocity ca. 10 times lower than the high temperature stage, is avoided due to the hydrogen removal through the membrane, which favors the CO conversion.An increase in the feed molar ratio to, e.g., 3 and 5 leads to a higher ratio between the MR volume and the one of the traditional process of 21% and 33%, respectively. This also has consequences on the reduction of the plant footprint.
In addition, around 90% of the H2 fed and produced by the reaction in the Pd–Ag MR was recovered in the permeate, when the MR operates at 15 bar and ca. 450 °C (outlet temperature). This stream, completely pure in H2, does not require any separation/purification, contrarily to the one exiting from the traditional process. Furthermore, other hydrogen is also contained in the retentate stream. MR retentate is concentrated (ca. 60–70%) and compressed in CO2, which can be easily captured, whereas the CO2 concentration of the residual stream exiting from the separation stage on the downstream of the traditional process does not exceed 60%.
List of symbols
| A | Membrane or heat exchange surface, m2 |
| d | Diameter, m |
| Cp | Species heat capacity, J mol−1 K−1 |
| Da | Damkoler number, - |
| E | Activation energy of hydrogen permeability, J mol−1 |
| F | Molar flow rate, mol s−1 |
| J | Permeation flux, mol m−2 s−1 |
| L | Length, m |
| P | Pressure, Pa |
| r | Reaction rate, mol m−3 s−1 |
| R | Ideal gas constant, J mol−1 K−1 |
| T | T/°C |
| U | Overall heat transfer coefficient, W m−2 K−1 |
| y | Molar fraction of the species ith, – |
| V | Volume, m3 |
| δ | Membrane thickness, m |
| ΔH | Heat of reaction, J mol−1 |
| ε | Catalytic bed voidage degree, - |
| μ | Viscosity, Pa·s |
| v | Stoichiometric coefficient, - |
| π | Permeance, mol m−2 s−1 Pa−0.5 |
| ρ | Density, kg m−3 |
| GHSV | Gas hourly space velocity |
| HT-WGS | High temperature water gas shift reactor |
| LT-WGS | Low temperature water gas shift reactor |
| MR | Pd-based membrane reactor |
| MREC | Membrane reactor equilibrium conversion |
| PEMFC | Polymer electrolyte membrane fuel cell |
| PSA | Pressure swing adsorption |
| TR | Traditional reactor |
| TREC | Traditional reactor equilibrium conversion |
| i | ith species referred to |
| Catalyst | Catalyst referred to |
| pellet | Catalyst pellet diameter referred to |
| Reaction | Membrane module stream on the reaction volume referred to |
| Feed | Membrane module inlet stream referred to |
| Furnace | Furnace referred to |
| Membrane | Membrane phase referred to |
| Permeation | Membrane module permeation stream referred to |
| Reaction | Membrane module stream on the reaction volume referred to |
| Shell | Membrane module shell side referred to |
| Sieverts | Sieverts’s law referred to |
| Sweep | Membrane module inlet stream on permeate side referred to |
| Tube | Membrane module tube side referred to |
Appendix
Mass balance
The mass balances for each species on the reaction and permeation side are given by eqn (4) and eqn (5), respectively:Reaction side:
 | (4) |
Permeation side:
 | (5) |
In the mass balance, the first term represents the convective flux variation of the i-th species along the reactor length, the second the reaction and the third the hydrogen permeation through the membrane. The mass balance equations of the permeate side consist of the same terms as at the reaction side, without that relative to the chemical reaction. The term of the permeating flux through the membrane has a positive sign because in this case the H2 enters in the permeation volume.
Energy balance
Eqn (6) and (7) give the energy balance for reaction and permeation sides, respectively, when the reaction happens in the annulus.Reaction side:
 | (6) |
Permeation side:
 | (7) |
The energy balance (eqn (6)) contains the heat produced by the chemical reaction, the convective flux of energy and the terms related to the heat exchanged with the furnace and permeation side. The heat produced by reactions and the exchange with the furnace is not present in the energy balance equation (eqn (7)) on the permeate side. In addition, a further term related to energy associated with the H2 permeating flux is also considered16 for completeness. The latter term gave a small contribution (∼5%).
Pressure drop
The pressure drop on the feed side was calculated using Ergun’s law, while a constant pressure is considered on the permeate side.Reaction side:
 | (8) |
Permeation side:
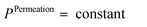 | (9) |
Table 5 reports the dimensionless variables used in the mass balance.

|
Dimensionless feed flow rate |

|
Dimensionless reaction rate |

|
Dimensionless length |
The kinetic equations used for the high temperature (eqn (2)) and the low temperature (eqn (3)) water gas shift reactions are those proposed by Keiski et al.4 and by Amadeo–Laborde 6, respectively.
 | (10) |
 | (11) |
Since the permeating driving force was created, increasing the feed pressure and maintaining constant atmospheric pressure on the permeate side, a very low sweep gas flow rate of hydrogen ( ) was used in the simulations for numerical reasons in order to fix the initial condition of the integration system.
) was used in the simulations for numerical reasons in order to fix the initial condition of the integration system.
References
- A. Tanksale, J. N. Beltramini and G. M. Lu, A review of catalytic hydrogen production processes from biomass, Renewable Sustainable Energy Rev., 2010, 14, 166–182 CrossRef CAS
.
- R. Kothari, D. Buddhi and R. L. Sawhney, Comparison of environmental and economic aspects of various hydrogen production methods, Renewable Sustainable Energy Rev., 2008, 12, 553–563 CrossRef CAS
.
- F. Bustamante, R. M. Enick, A. V. Cugini, R. P. Killmeyer, B. H. Howard, K. S. Rothenberger, M. V. Ciocco, B. D. Morreale, S. Chattopadhyay and S. Shi, High-Temperature Kinetics of the homogeneous Reverse Water–Gas Shift Reaction, AIChE J., 2004, 50(5), 1028–1040 CrossRef CAS
.
- R. L. Keiski, T. Salmi, P. Niemisto, J. Ainassaari and V. J. Pohjola, Stationary and transient kinetics of the high temperature water-gas shift reaction, Appl. Catal., A, 1996, 137, 349–370 CrossRef CAS
.
-
Ullman’s Encyclopedia of Industrial Chemistry, 5th Completely Revised Edition. Edited by Barbara Elvers, Stephen Hawkins, and William Russey. VCH: New York, 1995. ISBN 3-527-20126-2 Search PubMed
.
- N. E. Amadeo and M. A. Laborde, Hydrogen production from the low temperature water gas shift reaction: kinetics and simulation of the industrial reactor, Int. J. Hydrogen Energy, 1995, 20, 949–958 CrossRef CAS
.
- F. Barbir, PEM electrolysis for production of hydrogen from renewable energy sources, Sol. Energy, 2005, 78, 661–669 CrossRef CAS
.
- G. Barbieri, A. Brunetti, G. Tricoli and E. Drioli, An innovative configuration of a Pd-based membrane reactor for production of pure hydrogen. Experimental analysis of Water Gas Shift, J. Power Sources, 2008, 182, 160–167 CrossRef CAS
.
- E. Kikuchi, S. Uemiya, N. Sato, H. Inoue, H. Ando and T. Matsuda, Membrane reactor using microporous glass-supported thin film of palladium. Application to the water–gas shift reaction, Chem. Lett., 1989, 489–496 CrossRef CAS
.
- R. Dittmeyer, V. Hollein and K. Daub, Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium, J. Mol. Catal. A: Chem., 2001, 173, 135–84 CrossRef CAS
.
- S. Tosti, A. Basile, G. Chiappetta, C. Rizzello and V. Violante, Pd–Ag membrane reactors for water gas shift reaction, Chem. Eng. J., 2003, 93, 23–30 CrossRef CAS
.
- A. Brunetti, G. Barbieri, E. Drioli, T. Granato and K.-H. Lee, A porous stainless steel supported silica membrane for water gas shift reaction, Chem. Eng. Sci., 2007, 62(18–20), 5621–5626 CrossRef CAS
.
- X. Qi and M. Flytzani-Stephanopoulos, Activity and Stability of Cu-CeO2 Catalysts in High-Temperature Water-Gas Shift for Fuel-Cell Applications, Ind. Eng. Chem. Res., 2004, 43, 3055–3062 CrossRef CAS
.
- M. Flytzani-Stephanopoulos, X. Qi, S. Kronewitter, Water-gas Shift with Integrated Hydrogen Separation Process, Final Report 2004, DE-FG2600-NT40819 Search PubMed.
- J. Shu, B. P. A. Grandjean, A. Van Neste and S. Kaliaguine, Catalytic palladium-based membrane reactors: a review, Can. J. Chem. Eng., 1991, 69, 1036–1048 CrossRef CAS
.
-
F. A. Lewis, Palladium Hydrogen System, Academic Press, London, New York, 1967 Search PubMed
.
- S. Uemiya, N. Sato, H. Inoue, H. Ando and E. Kikuchi, The water–gas shift reaction assisted by palladium membrane reactor, Ind. Eng. Chem. Res., 1991, 30, 585–589 CrossRef CAS
.
- J. Shu, B. P. A. Grandjean, A. Van Neste and S. Kaliaguine, Catalytic palladium-based membrane reactors: a review, Can. J. Chem. Eng., 1991, 69, 1036–1048 CrossRef CAS
.
- A. Brunetti, G. Barbieri and E. Drioli, Upgrading of a syngas mixture for pure hydrogen production in a Pd-Ag membrane reactor, Chem. Eng. Sci., 2009, 64, 3448–3454 CrossRef CAS
.
- G. Marigliano, G. Barbieri and E. Drioli, Equilibrium
conversion for a palladium membrane reactor. Dependence of the temperature and pressure, Chem. Eng. Process., 2003, 42, 231–236 CrossRef CAS
.
- http://www.matthey.com .
- http://www.goodfellow.com .
- http://www.tokyo-gas.co.jp/techno/challenge/014_e.html .
- A. Brunetti, C. Caravella, G. Barbieri and E. Drioli, Simulation study of water gas shift in a membrane reactor, J. Membr. Sci., 2007, 306(1–2), 329–340 CrossRef CAS
.
- Johnson Matthey website www.platinum.matthey.com.
- F. Scura, G. Barbieri, E. Drioli, H2 for PEM-FC: effect of CO in the purification by means of Pd-based membranes, Desalination, 2006, 200(1-3), 239-241 – Euromembrane 2006 – September 24-28 Giardini Naxos, Taormina (Messina), Italy Search PubMed.
- G. Barbieri, A. Brunetti, T. Granato, P. Bernardo and E. Drioli, Engineering Evaluations of a Catalytic Membrane Reactor for the Water Gas Shift Reaction, Ind. Eng. Chem. Res., 2005, 44(20), 7676–7683 CrossRef CAS
.
- G. Barbieri, G. Marigliano, G. Perri and E. Drioli, Conversion-temperature diagram for a Palladium membrane reactor. Analysis of an endothermic reaction: methane steam reforming, Ind. Eng. Chem. Res., 2001, 40, 2017–2026 CrossRef CAS
.
- G. Q. Miller, J. Stöcker, Selection of a Hydrogen Separation Process, 1989 NPRA Annual Meeting held March 19-21, 1989, San Francisco, California (USA) Search PubMed.
- G. Q. Miller, J. Stöcker, Selection of a Hydrogen Separation Process, 4th European Technical Seminar on Hydrogen Plants, Lisbon (Portugal), Oct 2003 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |