Palladium-catalysed direct polyheteroarylation of di- or tribromobenzene derivatives: a one step synthesis of conjugated poly(hetero)aromatics†
Marya
Baloch
,
Reny Jacob
Roy
,
David
Roy
,
Kassem
Beydoun
and
Henri
Doucet
*
Institut Sciences Chimiques de Rennes, UMR 6226 CNRS-Université de Rennes "Catalyse et Organométalliques", 35042, Rennes, France. E-mail: henri.doucet@univ-rennes1.fr; Tel: +33 (0)2 23 23 63 84
First published on 12th October 2011
Abstract
The palladium catalysed polyheteroarylation of benzene, biphenyl, fluorene, naphthalene or antracene derivatives via C–H bond functionalisation allows the synthesis of a wide variety of functionalised conjugated poly(hetero)aromatics in only one step. This simple method provides a powerful tool to material chemists allowing to tune easily the physical properties of materials.
Introduction
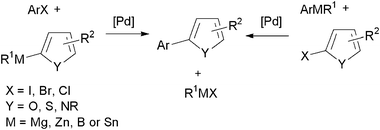
Molecular and polymeric semiconducting materials based on conjugated (hetero)aromatic rings have been extensively investigated for twenty years. For example, some arylthiophenes-containing polymers have been used as dye-sensitised solar cells. Such compounds also exhibit fluorescent or photoreversible photochromism properties.1 They are usually synthesized using Stille, Negishi or Suzuki cross-coupling reactions as the key step.2–4One possible approach for an easier and more direct access to polyheteroarylated arenes would obviously be the heteroarylationvia regioselective C–H bond functionalisation of heteroaromatics. In 1990, Ohta et al. reported the coupling of non functional thiophenes, furans or thiazoles with aryl halides, via a C–H bond activation/functionalisation, in moderate to good yields using 5 mol% Pd(PPh3)4 as the catalyst.5 Since these results, the so-called palladium-catalysed direct arylation of heteroaryl derivatives with aryl halides or triflates has proved to be a powerful method for the synthesis of arylated heterocycles.6–12 This reaction appears more attractive and useful than palladium catalysed Suzuki, Stille or Negishi cross-couplings, as no previous preparation of an organometallic derivative and its transmetallation product using B(OR)3, XSnR3 or ZnX2 is required (Scheme 1). Actually, one of the major drawbacks of these reactions is the limited substrate scope. To the best of our knowledge, only a few examples of palladium,13ruthenium or iridium14 catalysed direct arylation reactions using dihalobenzenes have been described and they employ quite large amount of catalyst or gave moderate yields. Moreover, to the best of our knowledge, no examples of palladium-catalysed direct coupling using 1,4-dihalonaphthalenes, 9,10-dihaloantracenes or 1,3,5-trihalobenzenes has been reported so far.
 | ||
| Scheme 1 | ||
We now report on the catalytic direct regioselective poly heteroarylation of di- or tribromoarenes with a wide variety of heteroaromatics, allowing to tune easily their physical properties.
Results and discussion
First, we attempted to promote the coupling of 2-i-butylthiazole with 1,4-dibromobenzene. For this reaction we employed as little as 0.5 mol% Pd(OAc)2/dppb [dppb: 1,4-bis(diphenylphosphino)butane] as the catalyst in the presence of KOAc as the base. We had previously observed that these conditions allow to catalyse very efficiently the direct arylation of several heteroaromatics (Scheme 2).15 We initially employed a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 2-i-butylthiazole and 1,4-dibromobenzene. In the course of this reaction, after 20 h, almost no coupling product 1 was detected. Only 2 arising from the 1,4-diheteroarylation of 1,4-dibromobenzene was detected, and it was isolated in 76%. As the formation of the monoheteroarylated benzene to give 1 might also be useful, we performed the reaction using a 1
1 ratio of 2-i-butylthiazole and 1,4-dibromobenzene. In the course of this reaction, after 20 h, almost no coupling product 1 was detected. Only 2 arising from the 1,4-diheteroarylation of 1,4-dibromobenzene was detected, and it was isolated in 76%. As the formation of the monoheteroarylated benzene to give 1 might also be useful, we performed the reaction using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of 2-i-butylthiazole and 1,4-dibromobenzene. However, the arylation of 1 to produce 2 seems to be much faster than the coupling with 1,4-dibromobenzene to give 1, and the ratio of mono- and diheteroarylated benzene 1:2 was 29
4 ratio of 2-i-butylthiazole and 1,4-dibromobenzene. However, the arylation of 1 to produce 2 seems to be much faster than the coupling with 1,4-dibromobenzene to give 1, and the ratio of mono- and diheteroarylated benzene 1:2 was 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71. Even in the presence of a 20
71. Even in the presence of a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the reactants, 1 was formed in only 60% selectivity and 53% yield. However, as 1,4-dibromobenzene is a very cheap reagent, and as its excess can be recycled this procedure might be useful. It should be noted that the use of 4-iodobromobenzene allows to prepare 1 in similar yield of 51% using only a 2
1 ratio of the reactants, 1 was formed in only 60% selectivity and 53% yield. However, as 1,4-dibromobenzene is a very cheap reagent, and as its excess can be recycled this procedure might be useful. It should be noted that the use of 4-iodobromobenzene allows to prepare 1 in similar yield of 51% using only a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of thiazole derivative and 4-iodobromobenzene. This is due to the easier oxidative addition of the palladium catalyst into the C–I bond than into the C–Br bond.
1 ratio of thiazole derivative and 4-iodobromobenzene. This is due to the easier oxidative addition of the palladium catalyst into the C–I bond than into the C–Br bond.
 | ||
| Scheme 2 | ||
The scope of this reaction was examined with other heteroaromatics using similar reaction conditions and a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of heteroaromatic and 1,4-dibromobenzene (Scheme 3). A furan, a thiophene, a pyrrole and an imidazole substituted at C2 led to the products 3–6 in 71–80% yields. Only the diheteroarylated benzene derivatives were detected. A regioselective arylation at C5 of the heteroaromatics was observed in all cases.
1 ratio of heteroaromatic and 1,4-dibromobenzene (Scheme 3). A furan, a thiophene, a pyrrole and an imidazole substituted at C2 led to the products 3–6 in 71–80% yields. Only the diheteroarylated benzene derivatives were detected. A regioselective arylation at C5 of the heteroaromatics was observed in all cases.
 | ||
| Scheme 3 | ||
The synthesis of mixed biheteroarylbenzenes is also possible from 1 (Scheme 4). For example, the reaction of 1 with 2-n-butyrylfuran gives 7 in 79% yield.
 | ||
| Scheme 4 | ||
From 1,3-dibromobenzene a similar reactivity than with 1,4- dibromobenzene was observed (Scheme 5). For these couplings, 3 equiv. of 2-substituted furan, thiophene, pyrrole, and also thiazole derivatives have been employed. In all cases, the diheteroarylated products 8–12 were formed in good to high yields. It should be noted that no formation of mono-heteroarylated 1,3-dibromobenzene was detected by GC analysis of the crude mixtures. The use of 2-chlorothiophene led to the C5 arylated thiophene to produce 10 in 66% yield. Under these reaction conditions, the aryl-chloride bond of the thiophene derivative appears to be unreactive.
 | ||
| Scheme 5 | ||
Then we attempted the diheteroarylation of 1,4′-dibromobiphenyl, and 2,7-dibromofluorene as such polyheteroarylated aromatics display useful physical properties.16 Both 1,4′-dibromobiphenyl and 2,7-dibromofluorene were found to give clean reactions, and in the presence of 3 equiv. of 2-i-butylthiazole, the diheteroarylated products 14 and 15 were selectively obtained in 81% and 83% yields (Schemes 6 and 7). It should be noted that in the presence of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of 2-i-butylthiazole and 1,4′-dibromobiphenyl, the monoheteroarylated biphenyl 13 was obtained in 88% selectivity and 77% yield. The arylation rates of 13 and 1,4′-dibromobiphenyl seems to be relatively similar allowing the formation of 13 in good yield. In the presence of 20 equiv. of 1,4′-dibromobiphenyl, 13 was almost exclusively obtained.
4 ratio of 2-i-butylthiazole and 1,4′-dibromobiphenyl, the monoheteroarylated biphenyl 13 was obtained in 88% selectivity and 77% yield. The arylation rates of 13 and 1,4′-dibromobiphenyl seems to be relatively similar allowing the formation of 13 in good yield. In the presence of 20 equiv. of 1,4′-dibromobiphenyl, 13 was almost exclusively obtained.
 | ||
| Scheme 6 | ||
 | ||
| Scheme 7 | ||
1,4′-Dibromobiphenyl was also coupled with 1-(furan-2-yl)butan-1-one, methyl 2-methylfuran-3-carboxylate and 2-acetylthiophene ethylene acetal using again 0.5 mol% Pd(OAc)2/dppb as the catalyst (Scheme 8). The desired products 16–18 were obtained in good yields of 72–83%.
 | ||
| Scheme 8 | ||
The 1,4-diheteroarylation of 1,4-dibromonaphthalene was expected to be more challenging, as this reactant is more hindered (Scheme 9).17,18 However, we observed that using similar reaction conditions, 0.5 mol% Pd(OAc)2/dppb as the catalyst at 150 °C, the target compounds 19–26 were obtained in good yields. A wide variety of heteroaromatics bearing useful functional groups have been employed. For example, thiophene 2-carbonitrile and 2-chlorothiophene led to 21 and 22 in 81% and 80% yields, respectively. A pyrrole bearing a formyl substituent at C2 was arylated regioselectively at C5 to produce 24 in 74% yield. In the presence of 2,5-dimethylisoxazole the arylation at C4 was observed to give 26 in 78% yield.
 | ||
| Scheme 9 | ||
Anthracenes substituted at C9 and C10 by heteroaromatics are also important building blocks in material chemistry.19 We have already reported that the direct arylation of some heteroaromatics proceed nicely with 9-bromoanthracene.17 The coupling of 9,10-dibromoanthracene with ten heteroaromatics was examined, and in all cases, the diheteroarylated anthracene derivatives 27–36 were obtained in 63–86% yields (Scheme 10). The reaction tolerates several functions at C2 or C3 of heteroaromatics such as chloro, acetyl, butyryl, ester or nitrile.
 | ||
| Scheme 10 | ||
Finally, we attempted the triheteroarylation of 1,3,5-tribromobenzene (Scheme 11). The synthesis in only one step of such compounds might be important for the preparation of electroluminescent or two-photon absorption materials.3c,20 It should also allow to prepare building blocks useful for the synthesis of dendrimers. For these couplings we employed 1 mol% PdCl(C3H5)(dppb) as the catalyst.8i,21 We initially examined the reactivity of furan derivatives. In all cases, the desired triheteroarylated benzene derivatives 37–41 were obtained in good yields. Only traces of mono or diheteroarylated benzenes were detected. It should be noted that even furfurylalcohol could be employed.22 The direct use of such heteroaromatics bearing an unprotected hydroxymethyl function is very useful in organic synthesis since it allows to avoid the protection/deprotection sequence. The coupling of several thiophene derivatives with 1,3,5-tribromobenzene also proceed in good yields. Again the coupling was found to be highly regioselective at C5 of all thiophene derivatives. In the presence of 2-chlorothiophene, no cleavage of the C–Cl bond occurs and 43 was obtained in 62% yield. 2-Acetylthiophene was also found to be a suitable coupling partner. The presence of such chloro or acetyl substituents on thiophene should allow the preparation of more complex conjugated structures presenting useful physical properties. The triheteroarylation of 1,3,5-tribromobenzene is not limited to the use of furans or thiophenes. The coupling with 1-methyl-2-formylpyrrole or 2-i-butylthiazole also gives the desired products 46 and 47 in 66% and 80% yields respectively.
 | ||
| Scheme 11 Direct coupling of heteroarenes with 1,3,5-tribromobenzene. | ||
We also performed UV-vis absorption and photoluminescent analysis of some the these compounds (see supplementary material†). UV-vis absorption of compounds 7, 15, 16 and 23 showed quite similar ìmax at ca. 330-360 nm; whereas the absorption maximum, of 36 was shifted about 30 nm at ca. 380 nm. The wavelength of emission was arround 410 nm for product 7, 420 nm for 16, 440 nm for 23, and 450 nm for 36.
Conclusions
In summary, we have demonstrated that polybromobenzenes can be coupled with heteroaromatics, using the simple Pd(OAc)2 precatalyst in the presence of the profitable influence of a diphosphine ligand (dppb) or using directly PdCl(C3H5)(dppb) as the catalyst. When using appropriate reaction conditions, dibromobenzenes, 1,4′-dibromobiphenyl, 2,7-dibromofluorene, 1,4-dibromonaphthalene or 9,10-dibromoanthracene were heteroarylated via C–H bond functionalisation of several heteroaromatics at C5. 1,3,5-Tribromobenzene was also successfully employed to prepare 1,3,5-tri(heteroaryl)benzenes. It should be noted that a wide range of heteroaromatics and functions such as formyl, acetyl, butyryl, ester, nitrile or chloro on the heteroaromatics are tolerated. Such functional group tolerance should allow the easy modification of structures to prepare materials presenting required physical properties. Moreover, this procedure employ a low loading of either a commercially available palladium source associated to a cheap diphosphine ligand, or an easily accessible catalyst.Experimental
General procedure
As a typical experiment, the reaction of the aryl bromide (1–20 mmol), heteroarene (1–5 mmol) and KOAc (2–5 mmol) at 150 °C during 20 h in DMAc (4–8 mL) in the presence of Pd(OAc)2/dppb 0.5 mol% or PdCl(C3H5)(dppb) 1 mol% (see schemes) under argon affords the coupling product after evaporation of the solvent and purification on silica gel.Preparation of the PdCl(C3H5)(dppb) catalyst:21
An oven-dried 40 mL Schlenk tube equipped with a magnetic stirring bar under argon atmosphere, was charged with [Pd(C3H5)Cl]2 (182 mg, 0.5 mmol) and dppb (426 mg, 1 mmol). 10 mL of anhydrous dichloromethane were added, then, the solution was stirred at room temperature for twenty minutes. The solvent was removed in vacuum. The yellow powder was used without purification. 31P NMR (81 MHz, CDCl3) δ = 19.3 (s).5-(4-Bromophenyl)-2-propylthiazole (1)
From 1,4-dibromobenzene (4.72 g, 20 mmol), 2-i-butylthiazole (0.141 g, 1 mmol) and KOAc (0.196 g, 2 mmol) in DMAc (8 mL) 1 was obtained in 53% (0.157 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.73 (s, 1H), 7.42 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 2.83 (d, J = 7.7 Hz, 2H), 2.14 (m, 1H), 1.08 (d, J = 7.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 170.4, 138.3, 137.5, 132.4, 130.9, 128.3, 122.1, 42.9, 30.1, 22.6. elemental analysis: calcd (%) for C13H14BrNS (296.23): C 52.71, H 4.76; found: C 52.89, H 4.87.1,4-Di(5-isobutylthiazol-2-yl)benzene (2)
From 1,4-dibromobenzene (0.236 g, 1 mmol), 2-i-butylthiazole (0.423 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 2 was obtained in 76% (0.271 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.86 (s, 2H), 7.53 (s, 4H), 2.85 (d, J = 7.7 Hz, 4H), 2.14 (m, 2H), 1.08 (d, J = 7.7 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 169.9, 137.8, 137.7, 131.2, 127.0, 42.6, 29.8, 22.3. elemental analysis: calcd (%) for C20H24N2S2 (356.55): C 67.37, H 6.78; found: C 67.24, H 6.95.1,4-Di(5-butyrylfuran-2-yl)benzene (3)
From 1,4-dibromobenzene (0.236 g, 1 mmol), 1-(furan-2-yl)butan-1-one (0.414 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 3 was obtained in 78% (0.273 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.86 (s, 4H), 7.28 (d, J = 3.6 Hz, 2H), 6.85 (d, J = 3.6 Hz, 2H), 2.87 (t, J = 7.7 Hz, 4H), 1.82 (sext., J = 7.7 Hz, 4H), 1.04 (t, J = 7.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 189.5, 156.7, 152.3, 130.0, 125.5, 119.3, 108.3, 40.6, 18.2, 14.1. elemental analysis: calcd (%) for C22H22O4 (350.41): C 75.41, H 6.33; found: C 75.50, H 6.21.1,4-Di[5-(2-methyl-[1,3]dioxolan-2-yl)-thiophen-2-yl]benzene (4)
From 1,4-dibromobenzene (0.236 g, 1 mmol), 2-acetylthiophene ethylene acetal (0.510 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 4 was obtained in 80% (0.331 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.58 (s, 4H), 7.19 (d, J = 3.7 Hz, 2H), 7.03 (d, J = 3.7 Hz, 2H), 4.08–3.97 (m, 8H), 1.83 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 147.5, 144.0, 134.1, 126.7, 125.8, 123.4, 107.8, 65.7, 28.2. elemental analysis: calcd (%) for C22H22O4S2 (414.54): C 63.74, H 5.35; found: C 63.61, H 5.18.1,4-Di(1-methyl-2-formylpyrrol-5-yl)benzene (5)
From 1,4-dibromobenzene (0.236 g, 1 mmol), 1-methyl-2-formylpyrrole (0.329 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 5 was obtained in 71% (0.207 g) yield. 1H NMR (300 MHz, CDCl3): δ 9.56 (s, 2H), 7.50 (s, 4H), 6.97 (d, J = 4.0 Hz, 2H), 6.34 (d, J = 4.0 Hz, 2H), 3.96 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 179.1, 142.6, 132.8, 130.7, 128.8, 123.9, 110.5, 33.9. elemental analysis: calcd (%) for C18H16N2O2 (292.33): C 73.95, H 5.52; found: C 73.99, H 5.61.1,4-Di(1,2-dimethylimidazol-5-yl)benzene (6)
From 1,4-dibromobenzene (0.236 g, 1 mmol), 1,2-dimethylimidazole (0.288 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 6 was obtained in 74% (0.197 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.29 (s, 4H), 6.86 (s, 2H), 3.58 (s, 6H), 2.47 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 146.5, 133.2, 130.0, 128.9, 126.2, 31.7, 13.9. elemental analysis: calcd (%) for C16H18N4 (266.34): C 72.15, H 6.81; found: C 72.20, H 6.97.1-(5-isobutylthiazol-2-yl)-4-(5-butyrylfuran-2-yl)benzene (7)
From 5-(4-bromophenyl)-2-propylthiazole (1) (0.296 g, 1 mmol), 1-(furan-2-yl)butan-1-one (0.276 g, 2 mmol) and KOAc (0.196 g, 2 mmol) in DMAc (4 mL) 7 was obtained in 79% (0.279 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.90 (s, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.60 (d, J = 8.0 Hz, 2H), 7.27 (d, J = 3.8 Hz, 1H), 6.80 (d, J = 3.8 Hz, 1H), 2.90 (d, J = 7.7 Hz, 2H), 2.86 (t, J = 7.7 Hz, 2H), 2.16 (m, 1H), 1.82 (sext., J = 7.7 Hz, 2H), 1.02–1.08 (m, 9H). 13C NMR (75 MHz, CDCl3): δ 188.7, 169.7, 156.1, 151.4, 137.5, 137.1, 131.7, 128.2, 126.3, 124.9, 118.5, 107.1, 42.0, 39.8, 29.2, 21.7, 17.4, 13.3. elemental analysis: calcd (%) for C21H23NO2S (353.48): C 71.35, H 6.56; found: C 71.24, H 6.65.1,3-Di(5-butyrylfuran-2-yl)benzene (8)
From 1,3-dibromobenzene (0.236 g, 1 mmol), 1-(furan-2-yl)butan-1-one (0.414 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 8 was obtained in 79% (0.276 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.15 (s, 1H), 7.79 (d, J = 7.8 Hz, 2H), 7.51 (t, J = 7.8 Hz, 1H), 7.28 (d, J = 3.6 Hz, 2H), 6.88 (d, J = 3.6 Hz, 2H), 2.88 (t, J = 7.7 Hz, 4H), 1.82 (sext., J = 7.7 Hz, 4H), 1.04 (t, J = 7.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 189.3, 156.5, 152.2, 130.2, 129.5, 125.5, 121.1, 119.0, 108.1, 40.4, 18.0, 13.9. elemental analysis: calcd (%) for C22H22O4 (350.41): C 75.41, H 6.33; found: C 75.32, H 6.21.1,3 Di(methyl 2-methyl-3-carboxylatefuran-5-yl)benzene (9)
From 1,3-dibromobenzene (0.236 g, 1 mmol), methyl 2-methylfuran-3-carboxylate (0.420 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 9 was obtained in 76% (0.269 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.91 (s, 1H), 7.55 (d, J = 7.8 Hz, 2H), 7.42 (t, J = 7.8 Hz, 1H), 6.97 (s, 2H), 3.85 (s, 6H), 2.66 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 164.0, 158.6, 151.0, 130.1, 128.8, 122.4, 118.4, 114.8, 105.6, 51.0, 13.5. elemental analysis: calcd (%) for C20H18O6 (354.35): C 67.79, H 5.12; found: C 67.70, H 5.20.1,3-Bis(5-chlorothiophen-2-yl)benzene (10)
From 1,3-dibromobenzene (0.236 g, 1 mmol), 2-chlorothiophene (0.355 g, 3 mmol) in DMAc (4 mL) 10 was obtained in 66% (0.205 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.62 (s, 1H), 7.46–7.34 (m, 3H), 7.12 (d, J = 3.8 Hz, 2H), 6.93 (d, J = 3.8 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 142.0, 134.3, 129.5, 129.4, 127.0, 124.8, 122.6, 122.4. elemental analysis: calcd (%) for C14H8Cl2S2 (311.25): C 54.02, H 2.59; found: C 54.09, H 2.70.1,3-Di(1-methyl-2-formylpyrrol-5-yl)benzene (11)
From 1,3-dibromobenzene (0.236 g, 1 mmol), 1-methyl-2-formylpyrrole (0.329 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 11 was obtained in 78% (0.228 g) yield. 1H NMR (300 MHz, CDCl3): δ 9.62 (s, 2H), 7.91 (s, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.51 (d, J = 7.8 Hz, 2H), 7.02 (d, J = 3.8 Hz, 2H), 6.36 (d, J = 3.8 Hz, 2H), 3.93 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 179.7, 143.1, 133.3, 131.7, 129.8, 129.2, 129.0, 124.3, 111.0, 34.4. elemental analysis: calcd (%) for C18H16N2O2 (292.33): C 73.95, H 5.52; found: C 73.84, H 5.60.1,3-Di(5-isobutylthiazol-2-yl)benzene (12)
From 1,3-dibromobenzene (0.236 g, 1 mmol), 2-i-butylthiazole (0.423 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 12 was obtained in 83% (0.296 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.83 (s, 2H), 7.61 (t, J = 1.6 Hz, 1H), 7.45–7.32 (m, 3H), 2.86 (d, J = 7.7 Hz, 4H), 2.12 (m, 2H), 1.00 (d, J = 7.7 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 169.5, 137.5, 137.2, 132.0, 129.1, 125.5, 124.2, 42.0, 29.3, 21.7. elemental analysis: calcd (%) for C20H24N2S2 (356.55): C 67.37, H 6.78; found: C 67.47, H 6.64.5-(4′-Bromobiphenyl-4-yl)-2-isobutylthiazole (13)
From 4,4′-dibromobiphenyl (1.248 g, 4 mmol), 2-i-butylthiazole (0.141 g, 1 mmol) and KOAc (0.196 g, 2 mmol) in DMAc (4 mL) 13 was obtained in 77% (0.286 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.89 (s, 1H), 7.65–7.55 (m, 6H), 7.48 (d, J = 8.2 Hz, 2H), 2.91 (d, J = 7.7 Hz, 2H), 2.18 (m, 1H), 1.06 (d, J = 7.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 169.9, 139.4, 139.2, 137.9, 137.8, 132.0, 131.1, 128.5, 127.4, 127.0, 121.8, 42.6, 29.8, 22.3. elemental analysis: calcd (%) for C19H18BrNS (372.32): C 61.29, H 4.87; found: C 61.40, H 4.69.4,4′-Di(5-isobutylthiazol-2-yl)biphenyl (14)
From 4,4′-dibromobiphenyl (0.312 g, 1 mmol), 2-i-butylthiazole (0.423 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 14 was obtained in 81% (0.350 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.67 (s, 2H), 7.45–7.35 (m, 8H), 2.90 (d, J = 7.7 Hz, 4H), 2.18 (m, 2H), 1.06 (d, J = 7.7 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 169.7, 139.6, 137.9, 137.6, 130.8, 127.2, 126.8, 42.4, 29.7, 22.2. elemental analysis: calcd (%) for C26H28N2S2 (432.65): C 72.18, H 6.52; found: C 72.29, H 6.41.2,7-Di(5-isobutylthiazol-2-yl)fluorene (15)
From 2,7-dibromofluorene (0.324 g, 1 mmol), 2-i-butylthiazole (0.423 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 15 was obtained in 83% (0.369 g) yield. 1H NMR (500 MHz, CDCl3): δ 7.90 (s, 2H), 7.78 (d, J = 8.0 Hz, 2H), 7.73 (s, 2H), 7.59 (d, J = 8.0 Hz, 2H), 3.98 (s, 2H), 2.92 (d, J = 7.2 Hz, 4H), 2.18 (m, 2H), 1.06 (d, J = 6.7 Hz, 12H). 13C NMR (125 MHz, CDCl3): δ 169.5, 144.2, 141.0, 138.9, 137.5, 130.4, 125.6, 123.2, 120.5, 42.6, 36.8, 29.8, 22.3. elemental analysis: calcd (%) for C27H28N2S2 (444.66): C 72.93, H 6.35; found: C 72.81, H 6.56.4,4′-Di(5-butyrylfuran-2-yl)biphenyl (16)
From 4,4′-dibromobiphenyl (0.312 g, 1 mmol), 1-(furan-2-yl)butan-1-one (0.414 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 16 was obtained in 72% (0.307 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 8.0 Hz, 4H), 7.71 (d, J = 8.0 Hz, 4H), 7.29 (d, J = 3.4 Hz, 2H), 6.84 (d, J = 3.4 Hz, 2H), 2.84 (t, J = 7.7 Hz, 4H), 1.80 (sext., J = 7.7 Hz, 4H), 1.06 (t, J = 7.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 189.2, 157.0, 152.0, 140.6, 128.8, 127.3, 125.4, 119.1, 107.6, 40.4, 18.0, 13.9. elemental analysis: calcd (%) for C28H26O4 (426.50): C 78.85, H 6.14; found: C 78.91, H 6.03.4,4-Di(methyl 2-methyl-3-carboxylatefuran-5-yl)biphenyl (17)
From 4,4′-dibromobiphenyl (0.312 g, 1 mmol), methyl 2-methylfuran-3-carboxylate (0.420 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 17 was obtained in 75% (0.322 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.72 (d, J = 8.0 Hz, 4H), 7.65 (d, J = 8.0 Hz, 4H), 6.94 (s, 2H), 3.88 (s, 6H), 2.69 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 163.8, 158.3, 150.9, 138.9, 128.5, 126.5, 123.5, 114.6, 105.1, 50.8, 13.3. elemental analysis: calcd (%) for C26H22O6 (430.45): C 72.55, H 5.15; found: C 72.48, H 5.24.4,4′-Di[5-(2-methyl-[1,3]dioxolan-2-yl)-thiophen-2-yl]biphenyl (18)
From 4,4′-dibromobiphenyl (0.312 g, 1 mmol), 2-acetylthiophene ethylene acetal (0.510 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 18 was obtained in 83% (0.407 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.70–7.62 (m, 8H), 7.23 (d, J = 3.7 Hz, 2H), 7.07 (d, J = 3.7 Hz, 2H), 4.20–4.00 (m, 8H), 1.86 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 146.6, 143.2, 139.2, 133.3, 127.0, 125.9, 124.9, 122.6, 107.0, 64.8, 27.3. elemental analysis: calcd (%) for C28H26O4S2 (490.64): C 68.54, H 5.34; found: C 68.35, H 5.39.1,4 Di(methyl 2-methyl-3-carboxylatefuran-5-yl)naphthalene (19)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), methyl 2-methylfuran-3-carboxylate (0.420 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 19 was obtained in 82% (0.331 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.50–8.40 (m, 2H), 7.78 (s, 2H), 7.65–7.55 (m, 2H), 7.02 (s, 2H), 3.91 (s, 6H), 2.75 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 164.0, 158.9, 150.3, 130.1, 127.5, 126.4, 125.3, 125.0, 114.7, 110.0, 51.0, 13.6. elemental analysis: calcd (%) for C24H20O6 (404.41): C 71.28, H 4.98; found: C 71.32, H 4.87.1,4-Di(5-methylthiophen-2-yl)naphthalene (20)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), 2-methylthiophene (0.294 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 20 was obtained in 84% (0.269 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.50–8.40 (m, 2H), 7.63–7.52 (m, 4H), 7.12 (d, J = 3.3 Hz 2H), 6.92 (d, J = 3.3 Hz 2H), 2.65 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 139.9, 138.9, 132.4, 131.8, 127.0, 126.9, 125.8, 125.7, 125.1, 14.9. elemental analysis: calcd (%) for C20H16S2 (320.47): C 74.96, H 5.03; found: C 75.04, H 5.11.1,4-Di(5-cyanothiophen-2-yl)naphthalene (21)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), thiophene 2-carbonitrile (0.327 g, 3 mmol) in DMAc (4 mL) 21 was obtained in 81% (0.277 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.20–8.15 (m, 2H), 7.75 (d, J = 3.8 Hz 2H), 7.65–7.61 (m, 2H), 7.60 (s, 2H), 7.31 (d, J = 3.8 Hz 2H). 13C NMR (75 MHz, CDCl3): δ 148.1, 137.2, 131.4, 131.2, 127.7, 127.4, 127.3, 125.2, 113.5, 109.7. elemental analysis: calcd (%) for C20H10N2S2 (342.44): C 70.15, H 2.94; found: C 70.31, H 3.10.1,4-Di(5-chlorothiophen-2-yl)naphthalene (22)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), 2-chlorothiophene (0.355 g, 3 mmol) in DMAc (4 mL) 22 was obtained in 80% (0.289 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.25–8.15 (m, 2H), 7.60–7.50 (m, 4H), 7.10–7.04 (m, 4H). 13C NMR (75 MHz, CDCl3): δ 140.4, 132.6, 132.5, 130.6, 128.0, 127.4, 127.3, 126.9, 126.4. elemental analysis: calcd (%) for C18H10Cl2S2 (361.31): C 59.84, H 2.79; found: C 59.64, H 2.70.1,4-Di[5-(2-methyl-[1,3]dioxolan-2-yl)-thiophen-2-yl]naphthalene (23)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), 2-acetylthiophene ethylene acetal (0.510 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 23 was obtained in 85% (0.395 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.40–8.32 (m, 2H), 7.59 (s, 2H), 7.58–7.51 (m, 2H), 7.17 (d, J = 3.5 Hz 2H), 7.15 (d, J = 3.5 Hz 2H), 4.12 (s, 8H), 1.92 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 146.8, 140.2, 131.6, 131.1, 126.5, 126.4, 125.5, 125.2, 123.4, 106.2, 64.1, 26.6. elemental analysis: calcd (%) for C26H24O4S2 (464.60): C 67.21, H 5.21; found: C 67.35, H 5.35.1,4-Di(1-methyl-2-formylpyrrol-5-yl)naphthalene (24)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), 1-methyl-2-formylpyrrole (0.329 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 24 was obtained in 74% (0.253 g) yield. 1H NMR (300 MHz, CDCl3): δ 9.70 (s, 2H), 7.72–7.65 (m, 2H), 7.60–7.50 (m, 4H), 7.14 (d, J = 4.0 Hz 2H), 6.44 (d, J = 4.0 Hz 2H), 3.76 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 180.2, 142.1, 133.2, 133.0, 130.8, 128.4, 127.8, 126.5, 124.7, 112.7, 34.6. elemental analysis: calcd (%) for C22H18N2O2 (342.39): C 77.17, H 5.30; found: C 77.04, H 5.41.1,4-Di(5-isobutylthiazol-2-yl)naphthalene (25)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), 2-i-butylthiazole (0.423 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 25 was obtained in 86% (0.349 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.20–8.10 (m, 2H), 7.74 (s, 2H), 7.55–7.45 (m, 4H), 2.92 (d, J = 7.2 Hz, 4H), 2.20 (m, 2H), 1.04 (d, J = 7.2 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 170.9, 141.4, 135.0, 132.2, 129.9, 127.9, 126.9, 125.8, 42.4, 29.8, 22.4. elemental analysis: calcd (%) for C24H26N2S2 (406.61): C 70.89, H 6.45; found: C 70.99, H 6.58.1,4-Di(3,5-dimethylisoxazol-4-yl)naphthalene (26)
From 1,4-dibromonaphthalene (0.286 g, 1 mmol), 3,5-dimethylisoxazole (0.291 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 26 was obtained in 78% (0.248 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.62–7.55 (m, 2H), 7.50–7.42 (m, 2H), 7.31 (s, 2H), 2.24 (s, 6H), 2.07 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 166.0, 159.5, 132.2, 127.9, 127.6, 126.3, 125.5, 114.4, 11.2, 10.3. elemental analysis: calcd (%) for C20H18N2O2 (318.37): C 75.45, H 5.70; found: C 75.31, H 5.59.9,10-Bis(5-butylfuran-2-yl)-anthracene (27)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), 2-n-butylfuran (0.372 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 27 was obtained in 76% (0.321 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.10–8.02 (m, 4H), 7.52–7.45 (m, 4H), 6.63 (d, J = 3.0 Hz, 2H), 6.36 (d, J = 3.0 Hz, 2H), 2.85 (t, J = 7.7 Hz, 4H), 1.78 (quint., J = 7.7 Hz, 4H), 1.56 (sext., J = 7.7 Hz, 4H), 1.03 (t, J = 7.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 157.6, 148.7, 131.7, 128.3, 127.0, 126.1, 113.5, 106.5, 30.8, 28.4, 22.7, 14.3. elemental analysis: calcd (%) for C30H30O2 (422.56): C 85.27, H 7.16; found: C 85.11, H 7.37.9,10-Bis(5-butyrylfuran-2-yl)-anthracene (28)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), 1-(furan-2-yl)butan-1-one (0.414 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 28 was obtained in 80% (0.360 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.93–7.85 (m, 4H), 7.55–7.47 (m, 4H), 7.46 (d, J = 3.5 Hz, 2H), 6.85 (d, J = 3.5 Hz, 2H), 2.91 (t, J = 7.7 Hz, 4H), 1.83 (sext., J = 7.7 Hz, 4H), 1.03 (t, J = 7.7 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 190.0, 154.1, 153.5, 131.1, 127.0, 126.8, 126.1, 117.7, 115.3, 40.6, 17.9, 14.0. elemental analysis: calcd (%) for C30H26O4 (450.53): C 79.98, H 5.82; found: C 80.10, H 6.02.9,10-Bis(5-acetoxymethylfuran-2-yl)-anthracene (29)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), furfuryl acetate (0.420 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 29 was obtained in 77% (0.350 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.00–7.92 (m, 4H), 7.53–7.45 (m, 4H), 6.77 (d, J = 2.7 Hz, 2H), 6.71 (d, J = 2.7 Hz, 2H), 5.25 (s, 4H), 2.15 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 171.2, 151.4, 150.6, 131.7, 127.9, 126.8, 126.6, 114.0, 112.3, 58.9, 21.5. elemental analysis: calcd (%) for C28H22O6 (454.47): C 74.00, H 4.88; found: C 74.14, H 5.01.9,10 Di(methyl 2-methyl-3-carboxylatefuran-5-yl)anthracene (30)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), methyl 2-methylfuran-3-carboxylate (0.420 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 30 was obtained in 79% (0.359 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.90–7.80 (m, 4H), 7.40–7.30 (m, 4H), 6.86 (s, 2H), 3.80 (s, 6H), 2.66 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 164.6, 160.0, 148.0, 131.2, 126.9, 126.3, 126.2, 114.7, 113.2, 51.5, 14.1. elemental analysis: calcd (%) for C28H22O6 (454.47): C 74.00, H 4.88; found: C 74.12, H 5.04.9,10-Bis(5-methylthiophen-2-yl)anthracene (31)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), 2-methylthiophene (0.294 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 31 was obtained in 84% (0.311 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.10–8.05 (m, 4H), 7.52–7.45 (m, 4H), 7.07 (d, J = 3.0 Hz, 2H), 7.03 (d, J = 3.0 Hz, 2H), 2.73 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 140.7, 136.0, 130.9, 130.0, 128.9, 126.3, 125.0, 124.8, 14.9. elemental analysis: calcd (%) for C24H18S2 (370.53): C 77.80, H 4.90; found: C 77.72, H 5.02.9,10-Bis(5-cyanothiophen-2-yl)anthracene (32)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), thiophene 2-carbonitrile (0.327 g, 3 mmol) in DMAc (4 mL) 32 was obtained in 74% (0.290 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 3.7 Hz, 2H), 7.85–7.70 (m, 4H), 7.60–7.45 (m, 4H), 7.27 (d, J = 3.7 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 146.3, 137.5, 130.8, 129.8, 127.8, 126.5, 125.7, 113.6, 111.2. elemental analysis: calcd (%) for C24H12N2S2 (392.50): C 73.44, H 3.08; found: C 73.52, H 3.00.9,10-Bis(5-acetylthiophen-2-yl)anthracene (33)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), 2-acetylthiophene (0.378 g, 3 mmol) in DMAc (4 mL) 33 was obtained in 63% (0.269 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.78 (d, J = 3.5 Hz, 2H), 7.70–7.62 (m, 4H), 7.30–7.22 (m, 4H), 7.09 (d, J = 3.5 Hz, 2H), 2.63 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 190.7, 147.8, 145.8, 132.7, 130.9, 130.7, 129.5, 126.3, 126.2, 26.9. elemental analysis: calcd (%) for C26H18O2S2 (426.55): C 73.21, H 4.25; found: C 73.04, H 4.31.9,10-Bis(5-chlorothiophen-2-yl)anthracene (34)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), 2-chlorothiophene (0.355 g, 3 mmol) in DMAc (4 mL) 34 was obtained in 83% (0.341 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.10–7.90 (m, 4H), 7.60–7.40 (m, 4H), 7.17 (d, J = 3.3 Hz, 2H), 7.02 (d, J = 3.3 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 137.5, 131.4, 130.9, 129.5, 129.0, 126.5, 126.4, 126.1. elemental analysis: calcd (%) for C22H12Cl2S2 (411.37): C 64.23, H 2.94; found: C 64.31, H 2.84.9,10-Bis[5-(2-methyl-[1,3]dioxolan-2-yl)thiophen-2-yl]anthracene (35)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), 2-acetylthiophene ethylene acetal (0.510 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 35 was obtained in 86% (0.442 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.00–7.90 (m, 4H), 7.50–7.40 (m, 4H), 7.28 (d, J = 3.5 Hz, 2H), 7.08 (d, J = 3.5 Hz, 2H), 4.17 (m, 8H), 1.98 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 148.6, 138.6, 131.3, 130.2, 129.3, 126.7, 125.7, 124.3, 107.3, 35.1, 27.5. elemental analysis: calcd (%) for C30H26O4S2 (514.66): C 70.01, H 5.09; found: C 70.10, H 5.02.9,10-Bis(5-isobutylthiazol-2-yl)anthracene (36)
From 9,10-dibromoanthracene (0.336 g, 1 mmol), 2-i-butylthiazole (0.423 g, 3 mmol) and KOAc (0.294 g, 3 mmol) in DMAc (4 mL) 36 was obtained in 78% (0.356 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.90–7.80 (m, 4H), 7.74 (s, 2H), 7.45–7.35 (m, 4H), 3.07 (d, J = 7.7 Hz, 4H), 2.29 (m, 2H), 1.12 (d, J = 7.7 Hz, 12H). 13C NMR (75 MHz, CDCl3): δ 171.8, 142.8, 131.6, 131.0, 126.4, 125.9, 125.7, 42.1, 29.5, 22.1. elemental analysis: calcd (%) for C28H28N2S2 (456.67): C 73.64, H 6.18; found: C 73.71, H 6.25.1,3,5-Tris(5-butylfuran-2-yl)-benzene (37)
1,3,5-tribromobenzene (0.315 g, 1 mmol), 2-n-butylfuran (0.620 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 37 was obtained in 73% (0.324 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.77 (s, 3H), 6.69 (d, J = 3.0 Hz, 3H), 6.12 (d, J = 3.0 Hz, 3H), 2.76 (t, J = 7.7 Hz, 6H), 1.74 (quint., J = 7.7 Hz, 6H), 1.52 (sext., J = 7.7 Hz, 6H), 0.99 (t, J = 7.7 Hz, 9H). 13C NMR (75 MHz, CDCl3): δ 156.7, 151.8, 131.8, 116.7, 106.8, 106.2, 30.2, 27.9, 22.3, 13.8. elemental analysis: calcd (%) for C30H36O3 (444.61): C 81.04, H 8.16; found: C 81.02, H 8.08.1,3,5-Tris(5-butyrylfuran-2-yl)-benzene (38)
1,3,5-tribromobenzene (0.315 g, 1 mmol), 1-(furan-2-yl)butan-1-one (0.690 g, 3 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 38 was obtained in 62% (0.301 g) yield. 1H NMR (300 MHz, CDCl3): δ 8.10 (s, 3H), 7.29 (d, J = 3.7 Hz, 3H), 6.96 (d, J = 3.7 Hz, 3H), 2.87 (t, J = 7.7 Hz, 6H), 1.83 (sext., J = 7.7 Hz, 6H), 1.03 (t, J = 7.7 Hz, 9H). 13C NMR (75 MHz, CDCl3): δ 189.2, 155.7, 152.3, 130.9, 121.4, 119.0, 108.7, 40.4, 17.9, 13.9. elemental analysis: calcd (%) for C30H30O6 (486.56): C 74.06, H 6.21; found: C 74.21, H 6.14.[1,3,5-Tris-(5-acetoxymethylfuran-2-yl)benzene (39)
1,3,5-tribromobenzene (0.315 g, 1 mmol), furfuryl acetate (0.700 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 39 was obtained in 74% (0.364 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.86 (s, 3H), 6.76 (d, J = 3.4 Hz, 3H), 6.54 (d, J = 3.4 Hz, 3H), 5.15 (s, 6H), 2.12 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 170.5, 153.9, 149.6, 131.5, 118.7, 112.7, 106.8, 58.2, 20.8. elemental analysis: calcd (%) for C27H24O9 (492.47): C 65.85, H 4.91; found: C 65.99, H 4.97.1,3,5-Tris-(5-hydroxymethylfuran-2-yl)benzene (40)
1,3,5-tribromobenzene (0.315 g, 1 mmol), furfurylalcohol (0.490 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 40 was obtained in 70% (0.256 g) yield. 1H NMR (300 MHz, MeOD): δ 7.88 (s, 3H), 6.82 (d, J = 3.2 Hz, 3H), 6.42 (d, J = 3.2 Hz, 3H), 4.60 (s, 6H). 13C NMR (75 MHz, MeOD): δ 156.1, 154.4, 133.2, 118.5, 110.7, 107.7, 57.6. elemental analysis: calcd (%) for C21H18O6 (366.36): C 68.85, H 4.95; found: C 68.69, H 5.08.1,3,5-Tris-(methyl 2-methyl-3-carboxylatefuran-5-yl)benzene (41)
1,3,5-tribromobenzene (0.315 g, 1 mmol), methyl 2-methylfuran-3-carboxylate (0.700 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 41 was obtained in 72% (0.354 g) yield. . 1H NMR (300 MHz, CDCl3): δ 7.68 (s, 3H), 6.94 (s, 3H), 3.86 (s, 9H), 2.67 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 164.2, 159.1, 150.8, 130.8, 117.5, 115.2, 106.4, 51.4, 13.9. elemental analysis: calcd (%) for C27H24O9 (492.47): C 65.85, H 4.91; found: C 65.68, H 5.08.1,3,5-Tris(5-methylthiophen-2-yl)benzene (42)23
1,3,5-tribromobenzene (0.315 g, 1 mmol), 2-methylthiophene (0.490 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 42 was obtained in 78% (0.285 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.64 (s, 3H), 7.22 (d, J = 3.0 Hz, 3H), 6.80 (d, J = 3.0 Hz, 3H), 2.62 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 141.1, 139.8, 135.6, 126.1, 123.3, 121.2, 15.3.1,3,5-Tris(5-chlorothiophen-2-yl)benzene (43)23
1,3,5-tribromobenzene (0.315 g, 1 mmol), 2-chlorothiophene (0.592 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 43 was obtained in 62% (0.265 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.49 (s, 3H), 7.14 (d, J = 3.8 Hz, 3H), 6.93 (d, J = 3.8 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 141.4, 135.2, 130.1, 127.2, 123.2, 121.9.1,3,5-Tris(5-acetylthiophen-2-yl)benzene (44)24
1,3,5-tribromobenzene (0.315 g, 1 mmol), 2-acetylthiophene (0.630 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 44 was obtained in 67% (0.301 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.85 (s, 3H), 7.71 (d, J = 3.9 Hz, 3H), 7.43 (d, J = 3.9 Hz, 3H), 2.61 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 190.5, 150.4, 144.2, 135.2, 133.3, 125.0, 124.1, 26.6.1,3,5-Tris[5-(2-methyl-[1,3]dioxolan-2-yl)-thiophen-2-yl]benzene (45)
1,3,5-tribromobenzene (0.315 g, 1 mmol), 2-acetylthiophene ethylene acetal (0.850 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 45 was obtained in 77% (0.448 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.68 (s, 3H), 7.25 (d, J = 3.6 Hz, 3H), 7.05 (d, J = 3.6 Hz, 3H), 4.08 (m, 12H), 1.84 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 147.3, 142.7, 135.4, 124.9, 123.3, 121.9, 107.0, 64.9, 27.3. elemental analysis: calcd (%) for C30H30O6S3 (582.75): C 61.83, H 5.19; found: C 61.94, H 5.08.1,3,5-Tris(1-methyl-2-formylpyrrol-5-yl)benzene (46)
1,3,5-tribromobenzene (0.315 g, 1 mmol), 1-methyl-2-formylpyrrole (0.548 g, 3 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 46 was obtained in 66% (0.263 g) yield. 1H NMR (300 MHz, CDCl3): δ 9.63 (s, 3H), 7.52 (s, 3H), 7.02 (d, J = 3.6 Hz, 3H), 6.39 (d, J = 3.6 Hz, 3H), 4.01 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 179.8, 142.1, 133.5, 132.3, 129.6, 124.3, 111.3, 34.5. elemental analysis: calcd (%) for C24H21N3O3 (399.44): C 72.16, H 5.30; found: C 72.28, H 5.41.1,3,5-Tris(2-isobutylthiazol-5-yl)benzene (47)
1,3,5-tribromobenzene (0.315 g, 1 mmol), 2-i-butylthiazole (0.705 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 47 was obtained in 80% (0.396 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.87 (s, 3H), 7.54 (s, 3H), 2.88 (d, J = 7.2 Hz, 6H), 2.18 (m, 3H), 1.01 (d, J = 7.2 Hz, 18H). 13C NMR (75 MHz, CDCl3): δ 170.5, 138.5, 137.0, 133.3, 124.1, 42.6, 29.8, 22.3. elemental analysis: calcd (%) for C27H33N3S3 (495.77): C 65.41, H 6.71; found: C 65.40, H 6.90.1,3,5-Tris(3,5-dimethylisoxazol-4-yl)benzene (48)
1,3,5-tribromobenzene (0.315 g, 1 mmol), 3,5-dimethylisoxazole (0.485 g, 5 mmol) and KOAc (0.490 g, 5 mmol) in DMAc (4 mL) 48 was obtained in 77% (0.280 g) yield. 1H NMR (300 MHz, CDCl3): δ 7.13 (s, 3H), 2.48 (s, 9H), 2.34 (s, 9H). 13C NMR (75 MHz, CDCl3): δ 165.6, 158.3, 131.6, 128.6, 115.8, 11.8, 11.0. elemental analysis: calcd (%) for C21H21N3O3 (363.41): C 69.41, H 5.82; found: C 69.40, H 6.04.Acknowledgements
Support provided from the ANR program for Sustainable Chemistry Development (ANR-09-CP2D-03 CAMELOT), Rennes Métropole and CNRS.References
- (a) G. Barbarella, M. Melucci and G. Sotgiu, Adv. Mater., 2005, 17, 1581 CrossRef CAS; (b) I. F. Perepichka, D. F. Perepichka, Handbook of Thiophene-based Materials: Applications in Organic Electronics and Photonics, Wiley, Chichester, 2009 Search PubMed.
- J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS.
- (a) X. M. Hong, H. E. Katz, A. J. Lovinger, B.-C. Wang and K. Raghavachari, Chem. Mater., 2001, 13, 4686 CrossRef CAS; (b) J. Wang, K. Liu, Y.-Y. Liu, C.-L. Song, Z.-F. Shi, J.-B. Peng, H.-L. Zhang and X.-P. Cao, Org. Lett., 2009, 11, 2563 CrossRef CAS; (c) B. L. Rupert, W. J. Mitchell, A. J. Ferguson, M. E. Koese, W. L. Rance, G. Rumbles, D. S. Ginley, S. E. Shaheen and N. Kopidakis, J. Mater. Chem., 2009, 19, 5311 RSC; (d) A. M. Fraind and J. D. Tovar, J. Phys. Chem. B, 2010, 114, 3104 CrossRef CAS; (e) T. Katagiri, Y. Shimizu, K. Terasaki, T. Yamao and S. Hotta, Org. Electron., 2011, 12, 8 CrossRef CAS.
- (a) J. J. Li, G. W. Gribble, Palladium in Heterocyclic Chemistry, Pergamon: Amsterdam, 2000 Search PubMed; (b) H. Doucet and M. Santelli, Synlett, 2006, 2001 Search PubMed.
- A. Ohta, Y. Akita, T. Ohkuwa, M. Chiba, R. Fukunaga, A. Miyafuji, T. Nakata, N. Tani and Y. Aoyagi, Heterocycles, 1990, 31, 1951 CrossRef CAS.
- (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (b) T. Satoh and M. Miura, Chem. Lett., 2007, 36, 200 CrossRef CAS; (c) B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949 CAS; (d) F. Bellina and R. Rossi, Tetrahedron, 2009, 65, 10269 CrossRef CAS; (e) L. Ackermann, R. Vincente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS; (f) J. Roger, A. L. Gottumukkala and H. Doucet, Chem Cat Chem, 2010, 2, 20 CAS; (g) C. Fischmeister and H. Doucet, Green Chem., 2011, 13, 741 RSC.
- (a) For recent examples of direct arylations of furans : M. Parisien, D. Valette, K. Fagnou and J. Org., Chem., 2005, 70, 7578 Search PubMed; (b) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Synthesis, 2008, 136 CrossRef CAS; (c) B. Liégaut, D. Lapointe, L. Caron, A. Vlassova, K. Fagnou and J. Org., Chem., 2009, 74, 1826 Search PubMed; (d) J. J. Dong, J. Roger, F. Požgan and H. Doucet, Green Chem., 2009, 11, 1832 RSC; (e) M. Ionita, J. Roger and H. Doucet, ChemSusChem, 2010, 3, 367 CAS.
- (a) For recent examples of direct arylations of thiophenes: K. Masui, A. Mori, K. Okano, K. Takamura, M. Kinoshita and T. Ikeda, Org. Lett., 2004, 6, 2011 CrossRef CAS; (b) E. David, S. Pellet-Rostaing and M. Lemaire, Tetrahedron, 2007, 63, 8999 CrossRef CAS; (c) H. A. Chiong and O. Daugulis, Org. Lett., 2007, 9, 1449 CrossRef CAS; (d) P. Amaladass, J. A. Clement and A. K. Mohanakrishnan, Tetrahedron, 2007, 63, 10363 CrossRef CAS; (e) M. Nakano, H. Tsurugi, T. Satoh and M. Miura, Org. Lett., 2008, 10, 1851 CrossRef CAS; (f) J. J. Dong, J. Roger and H. Doucet, Tetrahedron Lett., 2009, 50, 2778 CrossRef CAS; (g) J. Roger, F. Požgan and H. Doucet, Green Chem., 2009, 11, 425 RSC; (h) B. Liégault, I. Petrov, S. I. Gorlesky, K. Fagnou and J. Org., Chem., 2010, 75, 1047 Search PubMed; (i) F. Derridj, J. Roger, S. Djebbar and H. Doucet, Org. Lett., 2010, 12, 4320 CrossRef CAS.
- (a) For recent examples of direct arylations of pyrroles or indoles: F. Bellina, S. Cauteruccio and R. Rossi, Eur. J. Org. Chem., 2006, 1379 Search PubMed; (b) X. Wang, D. V. Gribkov, D. Sames and J. Org., Chem., 2007, 72, 1476 CAS; (c) N. Lebrasseur and I. Larrosa, J. Am. Chem. Soc., 2008, 130, 2926 CrossRef CAS; (d) Y. Fall, H. Doucet and M. Santelli, ChemSusChem, 2009, 2, 153 CrossRef CAS; (e) J. Roger and H. Doucet, Adv. Synth. Catal., 2009, 351, 1977 CrossRef CAS.
- (a) For recent examples of direct arylations of thiazoles: A. Mori, A. Sekiguchi, K. Masui, T. Shimada, M. Horie, K. Osakada, M. Kawamoto and T. Ikeda, J. Am. Chem. Soc., 2003, 125, 1700 CrossRef CAS; (b) A. Yokooji, T. Okazawa, T. Satoh, M. Miura and M. Nomura, Tetrahedron, 2003, 59, 5685 CrossRef CAS; (c) G. L. Turner, J. A. Morris and M. F. Greaney, Angew. Chem., Int. Ed., 2007, 46, 7996 CrossRef CAS; (d) L.-C. Campeau, M. Bertrand-Laperle, J.-P. Leclerc, E. Villemure, S. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3276 CrossRef CAS; (e) F. Derridj, J. Roger, S. Djebbar, H. Doucet and J. Organomet., Chem., 2009, 694, 455 CAS; (f) J. J. Dong, J. Roger, C. Verrier, T. Martin, R. Le Goff, C. Hoarau and H. Doucet, Green Chem., 2010, 12, 2053 RSC; (g) K. Beydoun and H. Doucet, ChemSusChem, 2011, 4, 526 CrossRef CAS.
- (a) For recent examples of direct arylations of imidazoles: F. Bellina, S. Cauteruccio, A. Di Flore and R. Rossi, Eur. J. Org. Chem., 2008, 5436 Search PubMed; (b) F. Bellina, S. Cauteruccio, A. Di Flore, C. Marchietti and R. Rossi, Tetrahedron, 2008, 64, 6060 CrossRef CAS; (c) J. Roger and H. Doucet, Tetrahedron, 2009, 65, 9772 CrossRef CAS.
- For recent examples of direct arylations of isoxazoles: Y. Fall, C. Reynaud, H. Doucet and M. Santelli, Eur. J. Org. Chem., 2009, 4041 Search PubMed.
- (a) For examples of palladium-catalysed direct arylations using dihalobenzenes or dihalobiphenyls: S. H. Mashraqui, M. Ashraf and S. G. Ghadigaonkar, Synlett, 2006, 2423 Search PubMed; (b) F. Bellina, S. Cauteruccio, A. Di Fiore and R. Rossi, Eur. J. Org. Chem., 2008, 5436 CrossRef CAS; (c) L. Ackermann, A. Althammer and P. Mayer, Synthesis, 2009, 3493 CrossRef CAS; (d) T. Kowada and K. Ohe, Bull. Korean Chem. Soc., 2010, 31, 577 CrossRef CAS; (e) A. Martins, D. A. Candito and M. Lautens, Org. Lett., 2010, 12, 5186 CrossRef CAS; (f) D. J. Schipper and K. Fagnou, Chem. Mater., 2011, 23, 1594 CAS.
- (a) For examples of ruthenium or iridium-catalysed direct arylations using dihalobenzenes: L. Ackermann, A. Althammer and R. Born, Synlett, 2007, 2833 Search PubMed; (b) B. Join, T. Yamamoto and K. Itami, Angew. Chem., Int. Ed., 2009, 48, 3644 CrossRef CAS; (c) L. Ackermann, R. Born and R. Vincente, ChemSusChem, 2009, 2, 546 CrossRef CAS; (d) L. Ackermann, R. Vincente, H. K. Potukuchi and V. Pirovano, Org. Lett., 2010, 12, 5032 CrossRef CAS; (e) N. Luo and Z. Yu, Chem. Eur. J., 2010, 16, 787 CrossRef CAS.
- (a) J. J. Dong and H. Doucet, Eur. J. Org. Chem., 2010, 611 CrossRef CAS; (b) L. Chen, J. Roger, C. Bruneau, P. H. Dixneuf and H. Doucet, Chem. Commun., 2011, 47, 1872 RSC.
- (a) B. C. Thompson, P. Schottland, K. Zong and J. R. Reynolds, Chem. Mater., 2000, 12, 1563 CrossRef CAS; (b) K. L. Woon, M. O'Neill, G. J. Richards, M. P. Aldred, S. M. Kelly and A. M. Fox, Adv. Mater., 2003, 15, 1555 CrossRef CAS.
- For palladium-catalysed direct mono-arylation using congested arylbromides: D. Roy, S. Mom, D. Lucas, H. Cattey, J.-C. Hierso and H. Doucet, Chem.–Eur. J., 2011, 17, 6453 CrossRef CAS.
- (a) For palladium-catalysed reactions using congested aryl halides: M. Feuerstein, H. Doucet and M. Santelli, Synlett, 2001, 1980 Search PubMed; (b) M. Feuerstein, F. Berthiol, H. Doucet and M. Santelli, Synthesis, 2004, 1281 CAS.
- (a) J. Pei, W.-L. Yu, J. Ni, Y.-H. Lai, W. Huang and A. J. Heeger, Macromol., 2001, 34, 7241 CrossRef CAS; (b) J. Ohshita, S. Kangai, Y. Tada, H. Yoshida, K. Sakamaki, A. Kunai, Y. Kunugi and J. Organomet, Chem., 2007, 692, 1020 CAS; (c) T. Kowada, Y. Matsuyama and K. Ohe, Synlett, 2008, 1902 CAS.
- (a) K. R. J. Thomas, J. T. Lin, Y.-T. Tao and C.-W. Ko, Chem. Mater., 2002, 14, 1354 CrossRef CAS; (b) S. A. Ponomarenko, S. Kirchmeyer, A. Elschner, B.-H. Huisman, A. Karbach and D. Drechsler, Adv. Funct. Mater., 2003, 13, 591 CrossRef CAS; (c) S. Liu, K. S. Lin, V. M. Churikov, Y. Z. Su, J. T. Lin, T.-H. Huang and C. C. Hsu, Chem. Phys. Lett., 2004, 390, 433 CrossRef CAS; (d) W. J. Mitchell, N. Kopidakis, G. Rumbles, D. S. Ginley and S. E. Shaheen, J. Mater. Chem., 2005, 15, 4518 RSC.
- T. Cantat, E. Génin, C. Giroud, G. Meyer, A. Jutand and J. Organomet, Chem., 2003, 687, 365 CAS.
- J. Roger, F. Pozgan and H. Doucet, Adv. Synth. Catal., 2010, 352, 696 CrossRef CAS.
- S. Kotha, D. Kashinath, K. Lahiri and R. B. Sunoj, Eur. J. Org. Chem., 2004, 4003 CrossRef CAS.
- N. P. Buu-Hoi, P. Jacquignon, F. Perin, M. Delcey and C. R. Hebd, Seances Acad. Sci., Ser. C, Sci. Chim., 1966, 262, 1237 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00370d/ |
| This journal is © The Royal Society of Chemistry 2011 |
