Incorporation of pyridazine rings in the structure of functionalized π-conjugated materials
Sylvain
Achelle
*a,
Nelly
Plé
b and
Alain
Turck
b
aSciences Chimiques de Rennes, UMR CNRS 6226, I.U.T. Lannion, rue Edouard Branly BP 30219, Lannion Cedex, France
bInstitut de Recherche en Chimie Organique Fine (IRCOF) UMR-CNRS 6014, INSA et Université de Rouen, BP08 76131 Mont-Saint-Aignan Cedex, France
First published on 19th August 2011
Abstract
Throughout the past few decades, the development of new π-conjugated organic compounds has received a lot of attention due to their potential applications ranging from liquid crystals, light-emitting and nonlinear optical applications to self-assembly and supramolecular chemistry. Most of these applications rely on electron transfer (ET) and excitation energy transfer (EET). Introduction in these conjugated structures of N-heterocycles such as the highly electronegative pyridazine ring appeared particularly interesting since the π-deficient character of the pyridazine, used as a dipolar moiety, favored the electron transfer. Moreover, the presence of nitrogen atoms with free electron pairs allows the pyridazine ring to act like as an effective and stable complexing agent for transition metal ions and to be used in supramolecular chemistry for the construction of highly sophisticated architectures. This review highlights recent π-conjugated materials including pyridazine ring and their applications in various fields.
1. Introduction
Diazines which belong to the most important heterocycles containing nitrogen are six-membered aromatics with two nitrogen atoms. Three different structures can be distinguished according to the relative position from the nitrogen atoms: pyridazine (1,2-diazine),1pyrimidine (1,3-diazine)2 and pyrazine (1,4-diazine).3 Whereas only a few natural 1,2-diazine derivatives are known,4 the pyridazine core can be found in a large range of biologically active structures such as herbicides5 and pharmaceutical drugs.6Over the past two decades, there has been a growing interest in the elaboration of π-conjugated organic compounds due their applications in various fields such as molecular wires, liquid crystals, electronic ad optoelectronic devices, non-linear optical (NLO) and two-photon absorption (TPA) materials.7 The synthesis of extended π-conjugated systems has been the key to provide organic materials with such properties. These compounds are often based on electronic transfer along the backbone of the molecule. Push-pull systems are of particular interest, generally, these compounds have symmetrical structure (D-π-D or A-π-A) or asymmetrical one (D-π-A), where A and D are electron acceptor or donor groups. The A and D substituents are linked through a π-conjugated spacer. The molecular properties of the chromophores depend on the strength of the “push-pull” effects which are function of the ability of D to provide electrons and of A to withdraw electrons along which the nature of the linkers.
The introduction of a heteoaryl moieties into π-extended systems is a way to modify and enhance useful properties of advanced “electro-optic” materials, heteroaromatics being particular useful where electron transport is necessary
The pyridazine with its highly π–deficient aromatic character is a good candidate to be incorporated as electron withdrawing part into push-pull scaffolds favoring the intramolecular charge transfer (ICT). The pyridazine, which is the most basic of the three diazines with a pKa = 2.3 allows protonation, hydrogen bond formation and chelation through the nitrogen atoms of the pyridazine ring, these are also of great importance: since such derivatives could be therefore used for formation of supramolecular assemblies and as sensors.
Two general methods are described in the literature for the synthesis of substituted pyridazine derivatives: The first method consists in the construction of the pyridazine ring from a 1,4-diketone and a hydrazine derivative,8 the second one is the functionalization of the pyridazine by nucleophilic substitutions,9 ortho-metallation10 and/or cross-coupling reactions.11 The advantage of the second way is a greater versatility and the use of various easily available starting materials that can be used as building blocks for the synthesis of π-conjugated structures. Cross-coupling reactions involving halogenated pyridazines constitute therefore an excellent synthetic pathway for the construction of such scaffolds. It should be noted that the π-electron-deficient character of the pyridazine ring makes easier the oxidative addition of palladium to a chlorine-carbon bond without the use of specialized and expensive ligands.12 Some cases of organometallic pyridazine derivatives that can be used as nucleophile in cross coupling reactions have also been described.13 Another synthetic way to obtain vinyl pyridazines is the condensation reaction of aldehydes with methylpyridazines.14
The aim of this paper is to review the various pyridazine derivatives used as building block in the synthesis of functionalized π-conjugated materials. This review is divided in five parts according to the domain of application of the structures. The first two parts will be dedicated to luminescence properties and non-linear optic phenomena. The third part will treat materials for charge transport such as field effect transistors and photovoltaic devices and the last two parts will review respectively liquid crystal structures and supramolecular assemblies.
2. Luminescence applications
Organic polymers and oligomers with good photoluminescent responses are promising candidates for photonic, electronic and optoelectronic applications.15 These systems consist in a π-conjugated backbone which alternate single and double (or triple) bonds along the chain. Poly(p-phenylenenevinylenes)16 are the most commonly used family of light emitting or light sensitive devices. Their electro-optical properties are known to depend on the chain length and the nature of substituents. The presence of nitrogen atoms in pyridazine ring, bearing a free electron pair, makes enable n→π* transitions in addition to the usual π→π* transitions observed in the electrons excitation of stilbenoid compounds.17 In ethanol, unsubstituted pyridazine shows the allowed π→π* transition at a λmax = 246 nm and the n→π* transition at λmax = 313 nm.18(E)-3-Styrylpyridazine has a strong red-shifted π→π* band at λmax = 290 nm and a shoulder for the n→π* transition at 350 nm in n-heptane.19Haroutounian et al. have synthesized 4′-hydroxystryrylpyridazines and studied their photophysical properties (Scheme 1).20 The UV spectra of compounds 1 and 2 in ethanol show under neutral conditions a long wavelength absorbance at 330–350 nm, which shifts sharply to the red in both acid and base (388–424 nm). The absorbance and fluorescence of these compounds demonstrate strong dependence on solvent polarity and pH, making them as potential probes for biological systems.
 | ||
| Scheme 1 | ||
Polarity, pH and ion-sensing fluorescent probes based on quadrupolar substituted 1,4-distyrylbenzenes with a pyridazine central core has been investigated by Schmitt et al. (Scheme 2).21 The compounds 3 and 4 have been obtained by aldol condensation. Whereas variations of the environment only slightly alter the electronic excitation spectra, the fluorescence spectra appear to be highly responsible: a positive solvatochromism is observed and the compounds are very sensitive toward protonation and highly responsible to iron (III) ions.
 | ||
| Scheme 2 | ||
The synthesis of various rod-like conjugated molecules with ethynyl and vinylpyridazines units has been performed by Hadad et al. using as key steps Sonogashira cross-coupling and aldol condensation reactions. The compounds 5–13 have highlighted interesting light-emitting properties due to internal charge transfer (Scheme 3).22
 | ||
| Scheme 3 | ||
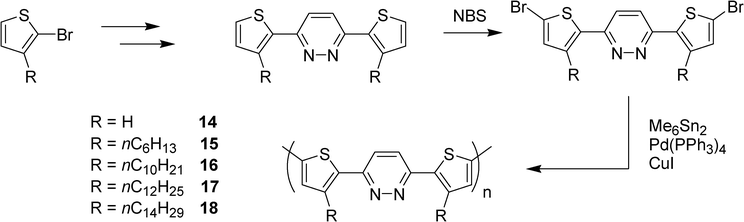
A series of π-conjugated copolymers with electron-donating thiophene or 3-alkylthiophene and electron-accepting pyridazine units have been synthesized by Yasuda et al. via an elaborated polycondensation utilizing hexamethylditin as the condensing reagent (Scheme 4).23 The copolymers 15–18 with long alkyl groups were soluble in organic solvents, and exhibited strong green photoluminescence (PL) (λabs 510–520 nm) with high quantum yields (ΦF > 0.5). The PL was sensible to protonation. The PL quantum yields of the copolymers 15–18 with a pyridazine core are higher than those of similar copolymers with a phenyl or a 3-pyridine unit. Furthermore, the polymers 14–18 were still photoluminescent in solid state.
 | ||
| Scheme 4 | ||
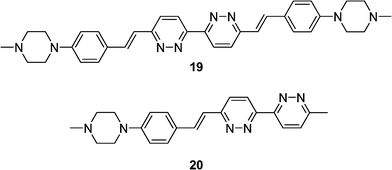
Highly fluorescent bipyridazines comprising a heterocyclic amine (piperazine) group were synthesized by Doet al. to give symmetric quadrupolar 19 and asymmetric dipolar 20 (Scheme 5).24 The fluorescence of these compounds was highly sensitive to solvent polarity (positive solvatochromism), giving a synthetic rainbow of emission in 10 different organic solvents. An organic sensitive poly(methylmethacrylate) film containing these compounds exhibited a visible sensitivity for the detection of solvents by their polarity upon exposure to organic solvent molecules. A patternable film sensor was fabricated by introducing a photo acid generator layer on a layer of dye 19.25 Fluorescent line patterns having a 10 μm width have been obtained by relatively weak power of UV light (0.4 mW/cm²) and high fluorescent contrast between the patterns.
 | ||
| Scheme 5 | ||
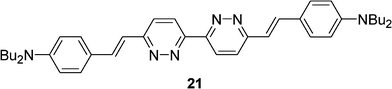
Lincker et al. reported in 2010 the design and synthesis of a new quadrupolar π-conjugated 3,3′-bipyridazine D-A-D ligand 21 (Scheme 6).26 Besides high fluorescence and pronounced solvatochromism, this compound exhibited an inherent electroactivity used to build an organic green light emitting device. The ability of this ligand to complex metal cations (CuI, NiII, PtII and IrIII) has been investigated to tune the electronic and optical properties.
 | ||
| Scheme 6 | ||
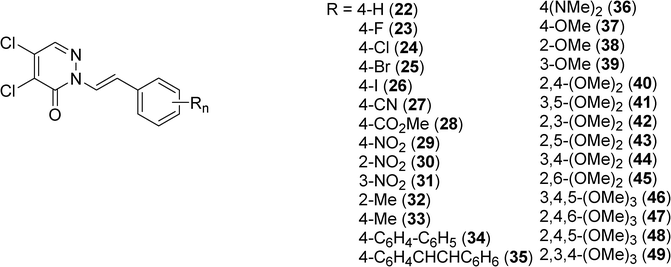
Kim et al. have studied the photophysical properties of several of trans-2-styrylpyridazin-3(2H)-ones (Scheme 7).27 They have highlighted that absorption and emission maxima of the compounds 22–49 respectively observed between 362–415 nm and 498–585 nm in CH2Cl2 were depending of the nature of the substituent and its position on the benzene ring. So a red shift was observed for electron-donating substituents at the para-position (compounds 36, 37, 40, 44, 46–49) and a blue shift for electron-withdrawing substituents (compounds 27–29). Some of these compounds are highly emissive with a fluorescence quantum yield up to 0.91.
 | ||
| Scheme 7 | ||
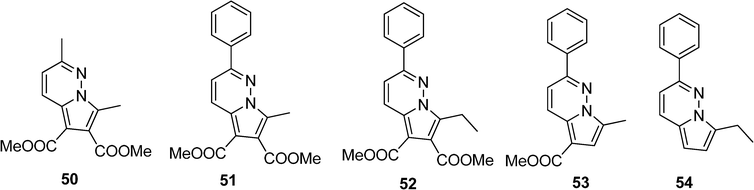
Pyrrolo[1,2-b]pyridazine derivatives, with aryl or methyl substituents on the pyridazinic ring and with alkyl, aryl and/or carbomethoxy on the pyrrolic ring, have been synthesized and studied by Vasilescu et al. (Scheme 8).28 The five compounds 50–54 have an intense fluorescence, with high quantum yields, as well in solution as in solid state. The presence of electron-withdrawing carbomethoxy groups (50–53) induces hypsochromic shifts whereas extension of the conjugation with a phenyl ring on the pyridazine (51–54) leads to bathochromic shifts with increasing quantum yield and lifetimes.
 | ||
| Scheme 8 | ||
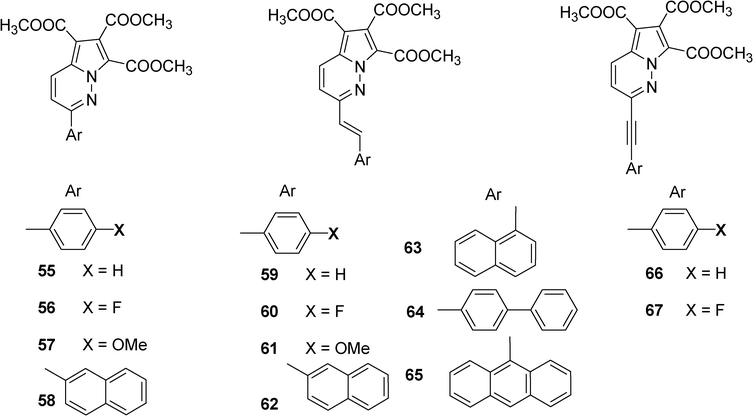
A series of pyrrolopyridazine derivatives with a core PPY (pyrrolo[1,2-b]pyridazine-5,6,7-tricarboxylic acid trimethylester) 55–67 have been synthesized by Swamy et al. and studied as blue organic luminophors (Scheme 9).29 These compounds have been obtained from 3-iodopyridazine or 3-methylpyridazine by cross coupling reaction and/or aldol condensation followed by 1,3-dipolarcycloaddition with dimethylacetylenedicarboxylate. The compounds 55–67 exhibit absorption band at 315–394 nm and emission band at 445–510 nm in DMSO. The derivative 56 showed a relative quantum yield as high as 0.9.
 | ||
| Scheme 9 | ||
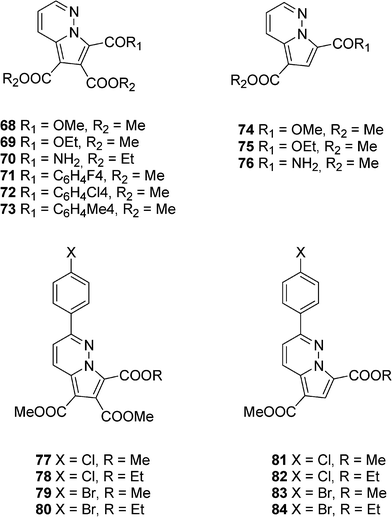
Other pyrrolopyridazine derivatives 68–84 have been described by Zbancioc et al. as blue organic luminophores (Scheme 10).30
 | ||
| Scheme 10 | ||
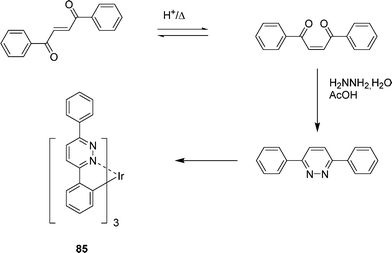
A green-light-emitting IrII complex (Ir(BPPya)3) 85 obtained from the 3,6-diphenylpyridazine has been described by Gao et al. (Scheme 11, Fig. 1).31 The complex 85 has been used as phosphorescent organic light emitting diode devices and appears as a promising material in terms of both efficiency and thermal stability. Based on 85, a device with a maximum external quantum efficiency of 14.6%, corresponding to a current efficiency of 52 cd A−1 and a power efficiency of 33.5 lm.W−1 has been obtained.
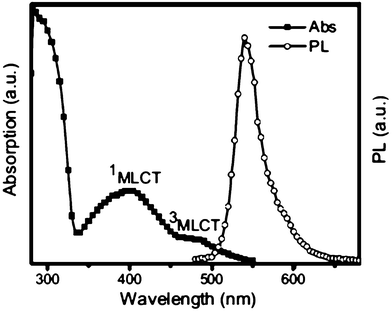 | ||
| Fig. 1 Ref. 32. UV-vis absorption and photoluminescence spectra of 85 in toluene (10−5M) (excitation @ 440 nm; MLCT: metal–ligand charge transfer). | ||
 | ||
| Scheme 11 | ||
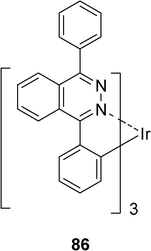
By changing the pyridazine ring by a phthalazine one, leading to iridium complex 86 with a CˆN=N structure (Scheme 12), the same authors have observed that the emission color can be tuned from green to saturated red.33
 | ||
| Scheme 12 | ||
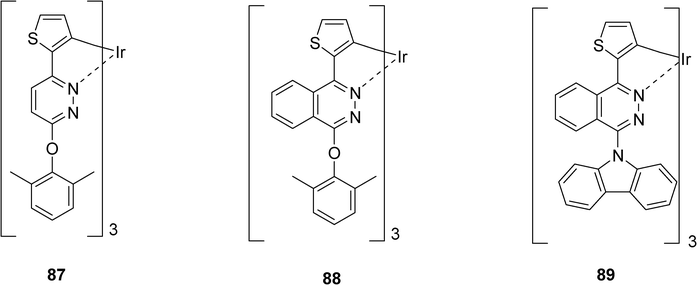
More recently, Fang et al. reported in 2010, the synthesis and characterization of three other iridium(III) complexes 87–89 with pyridazine and phthalazine derivatives as ligands (Scheme 13).34 The iridium complexes-based OLEDs fabricated by spin-coating technique exhibited promising performance. At a 4 wt% doping level and a practical luminance of 100 cd.m−2 using the compounds 87–89 as dopants, the external quantum efficiencies reached between 6.9 and 9.3% photons/electron.
 | ||
| Scheme 13 | ||
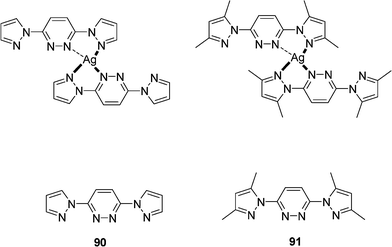
A series of silver complexes {[Ag(90(ClO4)]}n, {[Ag(91(NO3)]}n, {[Ag(90(PF6)]}n and {[Ag(91(ClO4).CH3OH]}n with the 3,6-bis(1-pyrazolyl)pyridazine as the ligand 90 and the 3,6-bis(3,5-dimethyl-1-pyrazolyl)pyridazine as the ligand 91 (Scheme 14) have been synthesized in the presence of different anions and characterized.35 The photoluminescence properties of these complexes have been investigated in the solid state at room temperature. These emissions at ca. 385 nm may be assigned to intra-ligand (n-π* and π–π*) emissions. A decrease of emission relative intensity is observed in case of complex {[Ag(90(PF6)]}n probably due to the obvious twisting mode of the ligand in the complex.
 | ||
| Scheme 14 | ||
Panigati et al. have synthesized and studied tetranuclear hydrido-carbonyl clusters of rhenium obtained by the reaction of electronically unsaturated tetrahedral cluster [Re4(μ3-H)4(CO)12] with pyridazine or phthalazine.36 The clusters obtained emits in the range of 580–645 nm, from MLCT excited states, with lifetimes comprised between 50–473 ns and emission quantum yield between 0.017 and 0.013. Other luminescent tricarbonyl rhenium(I) complexes with pyridazine or phthalazine ligand have been prepared and studied by the same team (Scheme 15).37 The complexes 92–97 exhibit also photoluminescence at room temperature in solution, with broad unstructured emission from MLCT excited states in the range 579–620 nm and with lifetimes between 20–2200 nm and quantum yields up to 0.12
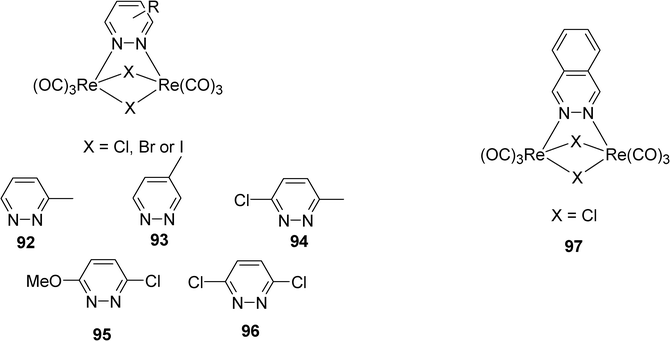 | ||
| Scheme 15 | ||
Redox driven chemiluminescent compounds based on thienyl and pyridazine systems have been studied by Asil et al. and Atilgan et al. (Scheme 16).38 The compounds 98–100 with a structure similar to luminol are soluble in aqueous and organic solvents and are highly sensitive towards Fe3+ ions which make them good candidate for forensic applications to detect blood stains (Fig. 2).
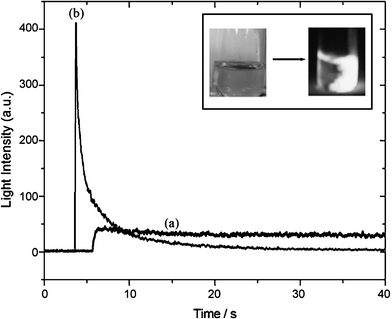 | ||
| Fig. 2 Ref. 39. The intensity of light emission by a CL reaction of 99 (1.0 × 10−5) dissolved in 0.1M NaOH and H2O2 (1.0 × 10−3) (a) without Fe3+ ion and (b) with Fe3+ ion (1.0 × 10−3). Inset: left side, Fe3+ solution; right side, the generated light after the addition of 99 dissolved in 0.1 M NaOH with H2O2. | ||
 | ||
| Scheme 16 | ||
The same team has also developed the first example of an electro-active chemiluminescent polymer bearing a pyridazine appendage (Scheme 17).40 The polymer 102 was obtained from monomer 101 by electrochemical means. Both monomer and polymer were shown to be (electro)chemiluminescent materials and are also highly sensitive towards Fe(III) ions which make them good candidates for use as analytical sensors and disposable electrodes.
 | ||
| Scheme 17 | ||
3. Non linear optical (NLO) applications
During the two last decades, organic nonlinear optical (NLO) materials have been extensively studied owing to their broad applications in the area of electronics and photonics.41 Among the numerous phenomena derived from the optical nonlinearity of organic molecules, simultaneous absorption of two photons has gained increasing attention over recent years. The two-photon absorption (TPA) opens the way for both improved and novel technological capabilities such as multiphoton microscopy,42 microfabrication,43 three-dimensional data-storage,44 optical power limiting45 and photodynamic therapy.46Three classes of compounds were found to be potential NLO chromophores; (1) dipolar type with a donor–bridge–acceptor (D-π-A) motif; (2) quadrupolar type with a D-π-D, A-π-A, D-π-A-π-D or A-π-D-π-A motif; (3) octupolar type with three-branched dipoles.
Whereas a lot of pyrimidine derivatives have been described with interesting TPA properties,47 literature describing such properties for pyridazine derivatives remain limited.
Among them, the compound 103 which belongs to a quadrupolar type and possess an arylamine donor and an acceptor pyridazine as the central core has been synthesized by Huang et al. (Scheme 18).48 TPA cross section (σ) measured by the up-converted fluorescence method with Ti:sapphire femtosecond laser excitation has a high σ value (1442 GM) and a particular large TPA cross section per mass σ/MW (1.97 GM/g) (Fig. 3).
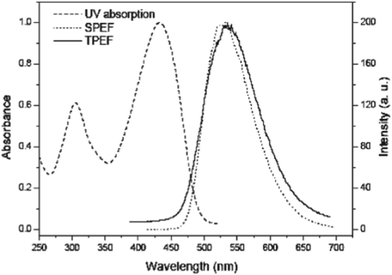 | ||
| Fig. 3 The UV absorption and single-photon-excited fluorescence (SPEF) spectra (c = 5.0 × 10−6 M) and the two-photon-excited fluorescence (TPEF) (c = 1.0 × 10−4 M) of 103 in DCM. | ||
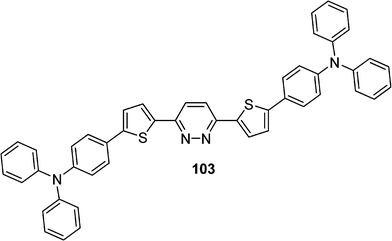 | ||
| Scheme 18 | ||
The copolymer 15 constituted of alternating pyridazine and 4,4′-dihexyl-2,2′-bithiophene units have been studied by Yamamoto et al.49 The author have shown that macromolecule 15 exhibits relatively limited TPA propertied with a cross section of 23 GM (measured by the up-converted fluorescence method).
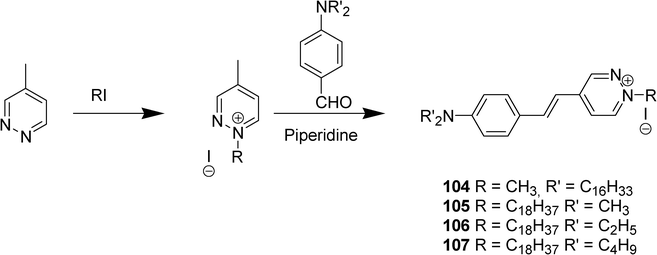
A series of conjugated compounds with a pyridazinium iodide moiety 104–107 have been synthesized by condensation of 4-methylpyridazinium iodide salt with various p-dialkylaminobenzaldehydes and characterized by Cheng et al. (Scheme 19).50 Strong second harmonic generation was obtained as well as Langmuir–Blodgett film forming properties. Among the factors which affect the second harmonic generation in the pyridazinium system, the high electron density around the second nitrogen in the pyridazine ring has been highlighted by the authors.
 | ||
| Scheme 19 | ||
Lithium salts of pyridazine have been studied theoretically as nonlinear optical molecules by Ma et al. (Scheme 20).51 Hyperpolarizability (β0) have been obtained at the second-order Møller–Plesset theory (MP2) The effect of the lithium salt of pyridazine (109, with H atom in position 4 on the pyridazine substituted by Li) on hyperpolarizability is observed as the β0 value is increased by about 170 times from 5 to 859 au. Another lithium salt (111) has been designed by doping two Na atoms into pyridazine. It has been found that these new lithium salts have a very large β0 value (1,412 × 106 au, 2.8 × 105 times larger than the pyridazine highlighting the sequence: 108 ≪ 109 ≪ 110 ≪ 111. This extraordinary high β0 value is currently a record and come from it small transition energy and large difference in the dipole moments between the ground state and the excited state.
 | ||
| Scheme 20 | ||
4. Materials for charge transport
Molecular electronics is a multidisciplinary research area that spans from the study of fundamental processes, such as electron transfer (ET) and excitation energy transfer (EET), to applied research, where the goal is fabrication of low-cost molecular sized electronic components. Electron and energy transfer reactions in covalently connected donor–bridge–acceptor assemblies (D–B–A) are dependent of the nature of A and D and of the electronic structure of the bridge.52In recent years, fabrication of electronic devices based on organic semiconductor materials has been fully investigated because of their application in low cost, large-area flexible display electronics.53 Among organic electronic components, organic field-effect transistors (OFETs) and photovoltaic cells are ones of the most important. To elaborate complementary circuit by using OFETs, not only p-type but also n-type transistors are necessary.54 The number of the latter currently available is still limited. In this context, the use of the strong electron withdrawing building block such as pyridazine ring for the synthesis of n-type semi-conductor is of high importance. Organic solar cells require conjugated polymers or oligomers with electron-withdrawing monomers.55Pyridazine derivatives appear therefore as interesting building blocks for the synthesis of such materials.
The first example of the use of pyridazine as building block for the synthesis of thin film field-effect transistor has been described by Yasuda et al.23 They described a series of π–conjugated (ABA)n-type polymers 112–116 constructed of electron-donating thiophene or 3-alkylthiophene (as the unit A) and electron accepting pyridazine (as the unit B) obtained by palladium-catalyzed homocoupling reaction (Scheme 21). In addition to their strong green photoluminescence, cyclic voltammetry revealed that the polymers were susceptible to both electrochemical p- and n-doping, and the n-doping peak appeared in the range of −2.15 to −2.28 V vs.Ag+/Ag. The polymers served as good materials for thin film field-effect transistor and gave a hole mobility of 3 × 10−3 cm2.V−1.s−1.
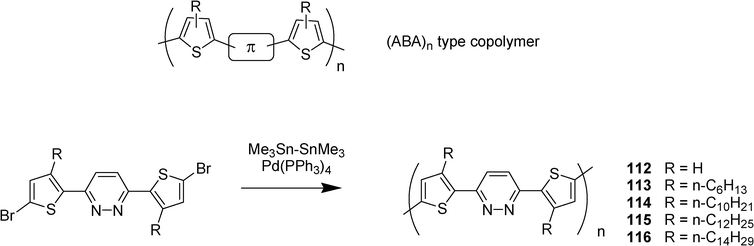 | ||
| Scheme 21 | ||
The synthesis, structural, electrochemical, optical and electronic structure properties of another series of diazine-thiophene semiconductor family has been reported.45 The compound 117 results from the incorporation of a pyridazine moiety into a oligothiophene, this compound has been obtained from 4,6-dichloropyridazine by two successive Stille cross-coupling reactions (Scheme 22). Electrochemical experiments carried out with the diazine-oligothiophenes revealed that incorporation of electron poor heteroaromatic rings into oligothiophene core significantly enhances electron affinity. However these materials in thin-film transistor are p-channel semiconductors. Experimental and computational analysis indicated that these materials essentially behave as π-extended bithiophenes. Nevertheless the authors suggest light-emitting transistor applications for these compounds which exhibit high solid state fluorescence efficiencies.
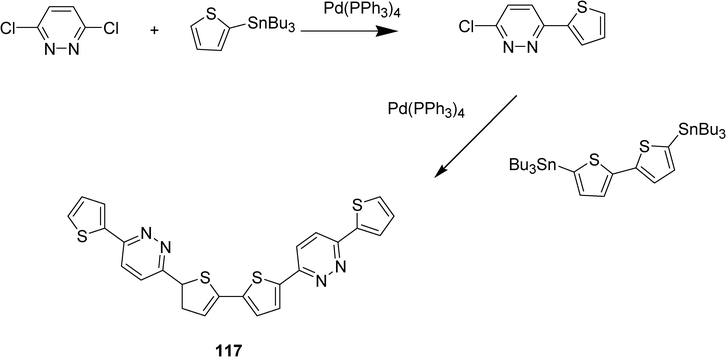 | ||
| Scheme 22 | ||
Pyridazine building blocks have also used for the design of artificial photosynthesis systems. Kropf et al. have studied the mono- and heteroleptic ruthenium complexes 118–130 containing substituted bipyridazine-glycol ligands (Scheme 23).56 The suitability as biomimetic model systems of the ruthenium complexes with two branches (type A: 118–121), three branches (type B: 127–130) and six branches (type C: 122–126) such as artificial photosynthesis systems are tested in two typical reactions: the sacrificial reduction of water and the reduction of CO2 to CH4. In the two cases; efficient results have been obtained with such complexes. Another series of ruthenium(II) polypyridazinyl complexes (131–134) with carboxylic ‘anchor’ have been synthesized as sensitizers for nanocrystalline-TiO2. “Graetzel”-type solar cells (Scheme 24).57 All sensitizers were successfully tested in the solar cell assembly.
 | ||
| Scheme 23 | ||
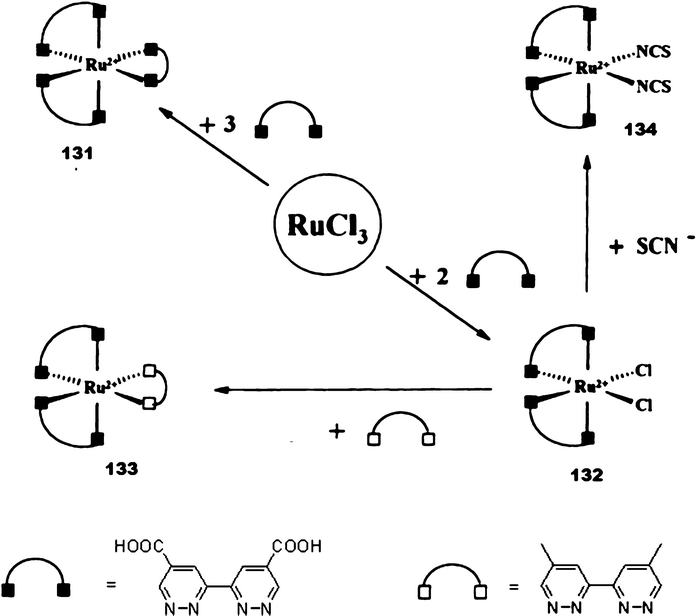 | ||
| Scheme 24 Ref. 58. | ||
Pyridazine-based monomers and related polymers have been synthesized by Gendron et al. for photovoltaic applications (Scheme 25).59 The structures 135–138 have been easily prepared from simple condensation reactions and ring closure procedures. Optimized HOMO, LUMO, and bandgap energy levels have been obtained. The resulting conjugated polymers have been tested in organic solar cells and exhibit power conversion efficiencies up to 0.5% for and active area of 1 cm2.
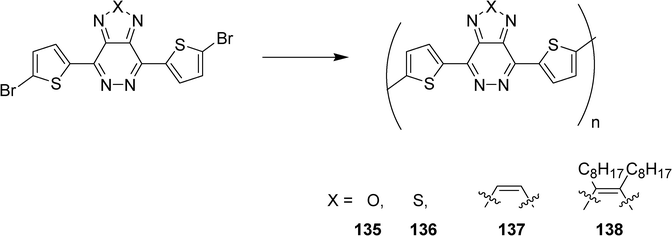 | ||
| Scheme 25 | ||
5. Liquid Crystals
Liquid crystal materials are fluid phases of matter where the constituent molecules are sufficiently disordered to be classified as a liquid and generate flow properties. Such materials present distinct phases of matter (mesophases) between the crystalline (solid) and isotropic (liquid) states. A proper molecular structure is required for molecules to be liquid crystals: a rigid aromatic core, strong dipoles and/or easily polarisable substituents and generally long flexible arms. Liquid crystals used in devices are anisotropic materials that exhibit two different dielectric constants parallel (ε//) and perpendicular (ε⊥) to the long molecular axis. For applications based on electrically controlled birefringence (EBC)60 or positive-contrast guest-host (GH),61 liquid crystals with a negative dielectric anisotropy (Δε = ε// − ε⊥ < 0) or with high ε⊥ values are required.62 Such material can be achieved by the introduction of lateral, polar groups such as F or CN into the mesogenic molecule.63 However lateral substituents such as cyano groups lead to a significant increase of the viscosity. Incorporation of 3,6-pyridazine moieties, whose lone pair electrons, located at the nitrogen atoms, form an orthogonal dipole to the long axis of the polyarylenes, is also an excellent mean to obtain liquid-crystalline compounds with negative dielectric anisotropy.64Paschke et al. have designed esters of 6-alkyloxypyridazine-3-carboxylic acid 139−141 (Scheme 26).65 Authors have shown that the introduction of nitrogen atoms with a pyridazine ring instead of a benzene or a pyridine one leads to significant change in the mesomorphic behaviour with a strong tendency to form smectic C phases.
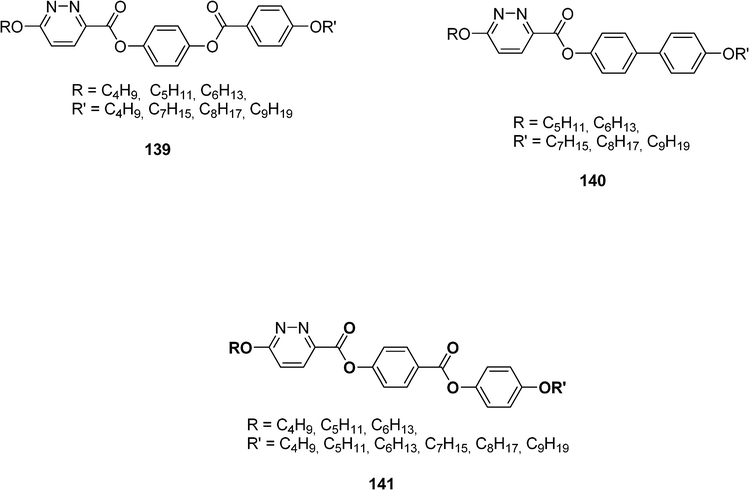 | ||
| Scheme 26 | ||
Synthesis of linear conjugated oligomers 142–145 with ethynylpyridazine units using Sonogashira and Suzuki cross-coupling reactions has been described by us (Scheme 27).66 The derivatives 142, 144 and 145 present interesting fluorescent and/or liquid crystal properties with smectic phases.
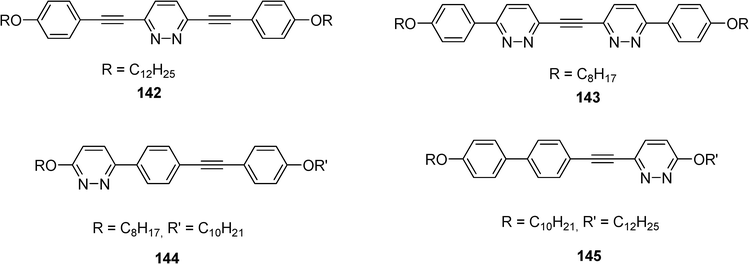 | ||
| Scheme 27 | ||
Other conjugated oligomers have been also developed by us with a trifluoromethylpyridazine moiety (Scheme 28).67 The structures 148–152 have been obtained from 6-aryl-3-chloro-4-trifluoromethylpyrazine by Suzuki cross-coupling reactions exhibit nematic mesophase.
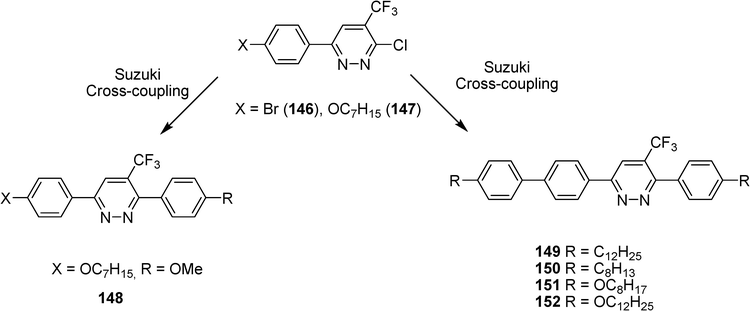 | ||
| Scheme 28 | ||
Wild et al. have studied the effect of the incorporation of the 2,6-bis-(4-pentylphenyl)pyridazine 153 (Scheme 29) as host material in the commercial nematic mixture E7 (Merck).68 An important increase of the flexoelectric effect has been observed allowing the use of such materials with important transversal dipole as switchable bistable liquid crystal (LCDs).
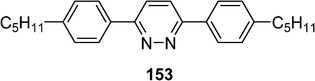 | ||
| Scheme 29 | ||
A variety of 3,6-bis(styryl)pyridazines (154) with 2, 4 or 6 alkoxy groups on the terminal benzene rings as pure (E,E) isomers were synthesized by Lifka et al. by using kinetically controlled Siegrist reaction (Scheme 30)69,17 The derivatives 154 have been obtained from 3,6-dimethylpyridazine and the corresponding azomethines by Siegrist reactions. As expected the transversal dipole moment of these calamitic compounds induces an extremely high tendency for self-organization in thermotropic mesophases nematic (N), smectic SA, SB, SC, SE, SF/I and cubic (Cub) The conjugated core structure represents moreover a chromophore with a high photosensitivity for (E,E) (E,Z) isomerization reactions: this property make the compounds interesting for optical imaging and switching techniques.
 | ||
| Scheme 30 | ||
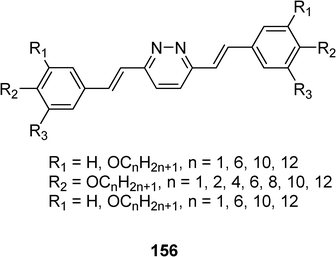
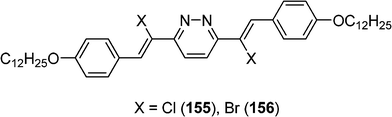
Halogenated 3,6-distyrylpyridazines derivatives 155 and 156 have been also synthesized by the same team (Scheme 31).70 For these compounds, liquid crystal and photoisomerisation properties are observed. An increase of the (E,Z)/(Z,Z) ratio to a certain limit leads to the breakdown of the smectic phase Sc. The nematic phase tolerates some more “wrong” configuration (E,Z) until the isotropic melt is generated. The authors described this process as the first photo-induced transformation between two mesophases.
 | ||
| Scheme 31 | ||
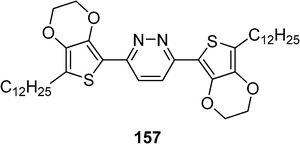
Liquid crystal compound 157 with an ethylenedioxythiophene-pyridazine-ethylenedioxythiophene core and two peripheral long alkyl chains has been prepared by Park et al. (Scheme 32).71 This molecule showed a highly ordered smectic phase (between 58 °C and 108 °C) due to strong intermolecular interactions (π–π stacking) of the D–A-D cores (Fig. 4).
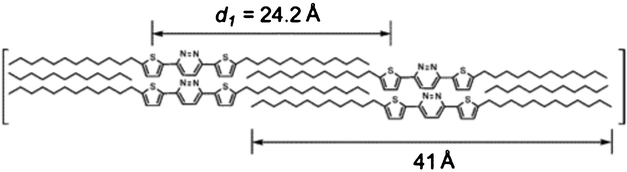 | ||
| Fig. 4 Ref. 72. A proposed molecular arrangement of the liquid crystal formed by 157. | ||
 | ||
| Scheme 32 | ||
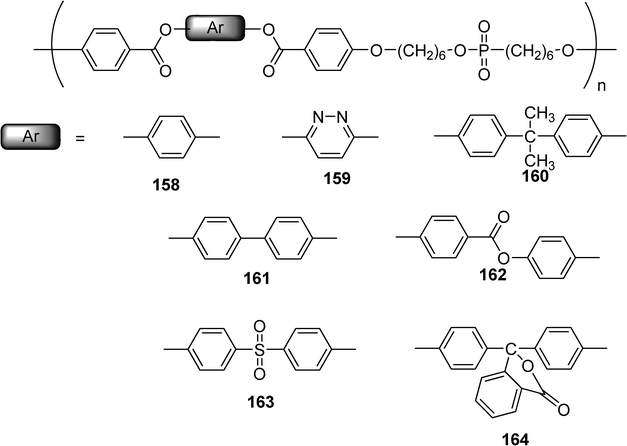
A series of main-chain liquid crystalline polyphosphates esters (158–164) with varying center aromatic substituents containing central unit of triad ester mesogen have been prepared and studied by Senthil et al. (Scheme 33).73 Among them, the structure 159 with a pyridazine moiety exhibited mesophase with increased stability compared to those of other derivatives, its temperature range up to more than 150 °C was depending of the length of the polymer chain.
 | ||
| Scheme 33 | ||
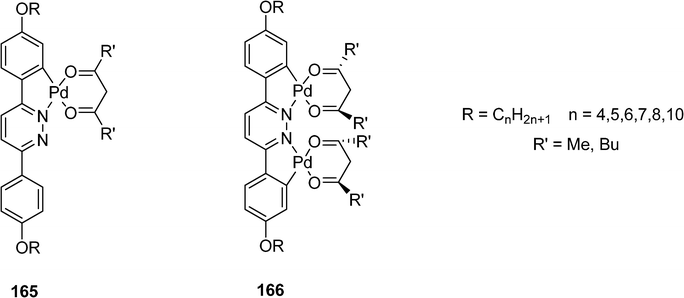
Metallomesogens 165 and 166 obtained by mono and diclyclopalladation of mesogenic pyridazines have been designed by Slater et al. (Scheme 34).74 The monometallated derivatives have a flat central core whereas the dimetallated derivatives are chiral with a sterically induced twist in the molecule. Smectic A phases are observed for the both type of derivatives with transition temperatures observed in the range 100–300 °C.
 | ||
| Scheme 34 | ||
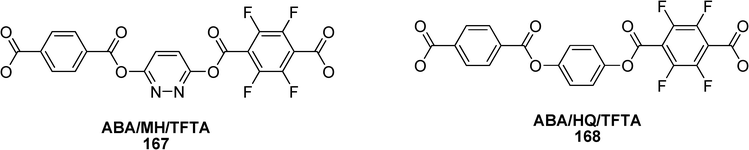
The effect of pyridazine structure on phase behaviour of thermotropic liquid crystalline copolyesters has been studied by Teoh et al. (Scheme 35).75 Authors have carried out the effects of maleic hydrazine (MH) and hydroquinone (HQ) on the liquid crystallinity and phase transition behaviour in the ABA/HQ/TFTA 168 and ABA/MH/TFTA 167 copolyesters (p-acetoxybenzoic acid (ABA) and tetrafluoroterephthalic acid (TFTA)) prepared by thin film polymerization. It has been demonstrated that the system 167 is energetically more favourable to mesophase formation than the system 168.
 | ||
| Scheme 35 | ||
6. Supramolecular assemblies
In the last twenty-five years, a generation of nanomolecular architectures has developed at a tremendous rate. This expansion has been driven by the interest of these complex systems in the scientific fields of chemistry (molecular recognition), biology (translocation of drugs across membranes) and materials science (construction of macroscopic architectures and devices on the molecular level). Elaboration of nanosized molecular structures is due to reversible bond formation capacity of self-assembling systems. This strategy has made successful use of reversible π–π stacking, H-bonding and metal-ion-ligand coordination interactions. The supramolecular chemistry of pyridazine is particularly rich due to the ability of this heterocycle to complex metal cations.Carlucci et al. have studied the complexation of the pyridazine with silver salts.76 Various polymeric helical species have been obtained using nitrate, triflate or tetrafluoroborate silver(I) salts (Fig. 5).
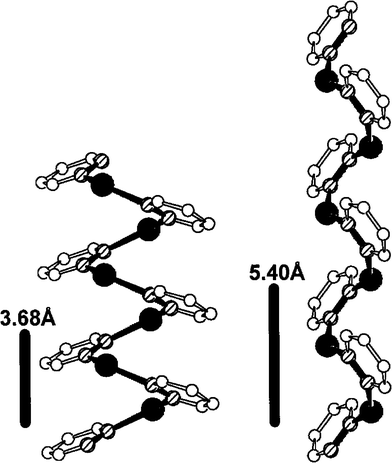 | ||
| Fig. 5 Ref. 77. View of helical backbone of polymeric helical motifs obtained with pyridazine and Ag(NO2) and Ag(SO3CF3). | ||
An oligoheterocyclic with a pyridine-pyridazine sequences comprising thirteen heterocycles has been obtained by Cuccia et al. using the Potts methodology (Scheme 36).78 Due to a strong favored transoid conformation of the α,α′ interheterocyclic bonds, this compound exhibits a pre-organized helical form leading to self-assembled supramolecular stacks presenting twelve heterocycles per turn with an outside diameter of about 25 Å and a central cavity of about 8 Å (Fig. 6). The tubular (helical) formation stacks with hollow cores of finite size opens ways for the design of functional polymolecular materials for multichannel ion-active devices. Mechanically stable two-dimensional Langmuir–Blodgett and cast thin films are also obtained with compound 169.
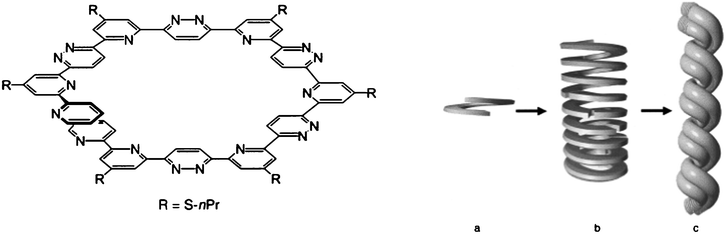 | ||
| Fig. 6 Ref. 79. Schematic representation of the hierarchical self-assembly of compound 169. The self-organized lock-washer structure (a) of 169 is proposed to self-assemble to form protofibrils or filaments (b), and fibrils (c). | ||
 | ||
| Scheme 36 | ||
Various 3,6-bis(2-pyridyl)-pyridazine (bppn) derivatives (170–172) have been fully investigated as metal-coordinating ligands resulting in grid-like metal complexes (Fig. 7). Such structures have been obtained with copper(I),80silver(I)81 and nickel(II)82 ions. Weissbush et al. have studied the 3,6-bis[2-(6-phenylpyridine)pyridazine as ligand.83 This structure can lead to oriented crystalline monolayers and bilayers of 2 × 2 silver(I) grid architectures. Such self-assembled structure may lead to organometallic composite that contain homodisperse semiconducting or conducting particles with new optoelectronic properties.
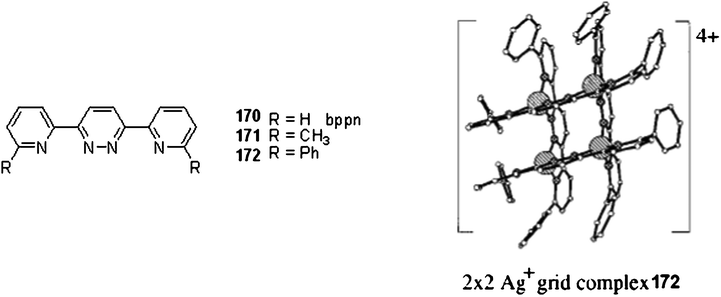 | ||
| Fig. 7 Ref. 84. 2 × 2 Ag+ grid complex 172. | ||
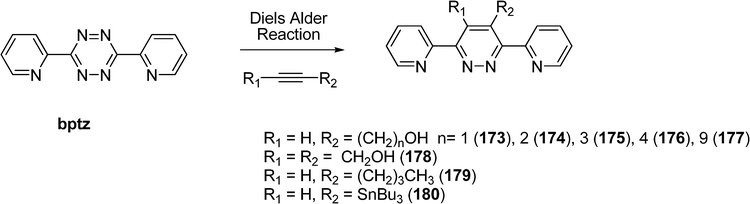
Other [2 × 2] grid-like metal complexes have been obtained with copper(I) and silver(I) ions and functionalized 3,6-di(2-pyridyl)pyridazines (173–180) obtained via an inverse demand Diels–Alder reaction between the corresponding 3,6-di(2-pyridyl)-1,2,4,5-tetrazine (bptz) and various alkynes (Scheme 37).85
 | ||
| Scheme 37 | ||
Constable et al. have studied the self-assembly resulting from reaction of various 4-substituted-3,6-di(2-pyridyl)pyridazine 181–187 (Scheme 38) with silver(I) tetrafluoroborate.86 A pentanuclear centered-tetrahedral silver complex has been obtained with the compound 181.87 As indicated on Fig. 8, two tetrahedra are formed, one described by the four silicon atoms and the second by the four outermost silver centers. Investigation of the role played by other sterically demanding substituents has been carried out with compounds 182–187 in the self-assembly of complexes obtained with 1 equivalent of silver(I) tetrafluoroborate in acetonitrile.88 Whereas the crystalline products of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reactions between ligand 182 and 187 and silver(I) tetrafluoroborate lead to an [Ag(L)2][BF4] structure (Fig. 9), a [Ag(L)2][BF4]2 structure is observed for ligands 183–186 (Fig. 10). The packing in [Ag2(185)2][BF4]2·2.6MeCN complex is dominated by cation••••anion interactions in contrast to the packing in [Ag(188)2][BF4] which exhibits no close interaction Ag••••Ag contacts and only one short Ag••••Cpy contact.
1 reactions between ligand 182 and 187 and silver(I) tetrafluoroborate lead to an [Ag(L)2][BF4] structure (Fig. 9), a [Ag(L)2][BF4]2 structure is observed for ligands 183–186 (Fig. 10). The packing in [Ag2(185)2][BF4]2·2.6MeCN complex is dominated by cation••••anion interactions in contrast to the packing in [Ag(188)2][BF4] which exhibits no close interaction Ag••••Ag contacts and only one short Ag••••Cpy contact.
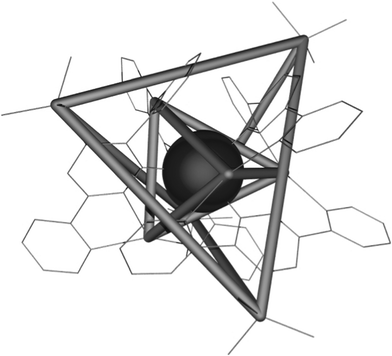 | ||
| Fig. 8 Ref. 87. The relationship between the two tetrahedra described by the four silicon atoms and the four outermost silver centres of 181. | ||
![Ref. 89. Molecular structure of the [Ag(187)2]+ in [Ag(187)2]-[BF4].](/image/article/2011/RA/c1ra00207d/c1ra00207d-f9.gif) | ||
| Fig. 9 Ref. 89. Molecular structure of the [Ag(187)2]+ in [Ag(187)2]-[BF4]. | ||
 | ||
| Scheme 38 | ||
Hoogenboom et al. have described the synthesis of a hydroxyl-functionalized 3,6-bis(2-pyridyl)pyrazine ligand from 3,6-bis-(2-pyridyl)tetrazine (bptz) and 5-hexyn-1-olviaazide-alkyne cycloaddition (‘click chemistry’) leading to the formation of the derivative 188 (Scheme 39). The ‘supramolecular click’ reaction of 188 with copper(I) ions allowed to obtain star-shaped polymeric [2 × 2] grid-like copper(I) complexes (Fig. 11).90 Starting from 188, this same team has also described the formation of supramolecular star-shaped polymers by self-assembly of polymeric precursors poly(L-lactide)91 and poly(ethyleneglycol).92
![Ref. 90. Star-shaped polymeric [2 × 2] grid-like copper(I) complexes of 188 with copper(I) ions.](/image/article/2011/RA/c1ra00207d/c1ra00207d-f11.gif) | ||
| Fig. 11 Ref. 90. Star-shaped polymeric [2 × 2] grid-like copper(I) complexes of 188 with copper(I) ions. | ||
 | ||
| Scheme 39 | ||
Manzano and coworkers have studied the building of [2 × 2] metallic grid with copper(I) cations and 3,6-bis(3,5-dimethylpyrazol-1-yl)pyridazine 189 (Scheme 40).93 Contrary to supramolecular assemblies obtained with a pyrimidine central core instead of the pyridazine one, no encapsulation of the BF4− or PF6− counteranions has been observed due to the size of the cavities generated in the grid which were too small.
 | ||
| Scheme 40 | ||
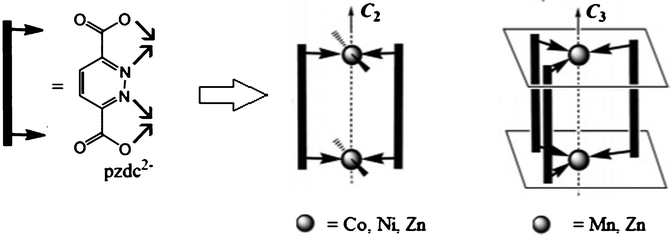
Sun et al. have described the reaction of the pyridazine-3,6-dicarboxylate (pzdc) a bis(bidentate) ligand with appropriate metal chlorides in the presence/absence of KCl, NaCl and NH4Cl.94 The structures of the coordination compounds of pyridazine-3,6-dicarboxylate (pzdc) with Mn(II), Co(II), Ni(II) and Zn(II) have been reported. All Mn(II) species contain the binuclear trigonal prism anion [M2(pzdc)3]2− which co-assemble with different cations [Mn(H2O)6]2+, NH4+, K+, Na+, to generate coordination or hydrogen-bonded networks. The products for Co(II) and Ni(II) are side-by-side compounds self-assembled into 3D hydrogen-bonded networks. Whereas the structure of Zn(II) depends of presence/absence of KCl, NaCl and NH4Cl and are analogous to the Mn(II) species (Fig. 12). The structure of lead(II) diaqua 3,6-dicarboxylatopyridazine has been studied by Sabanska et al. and consists of columns of lead atoms linked by tetradentate pyridazine-3,6-dicarboxylate in a spiral array.95
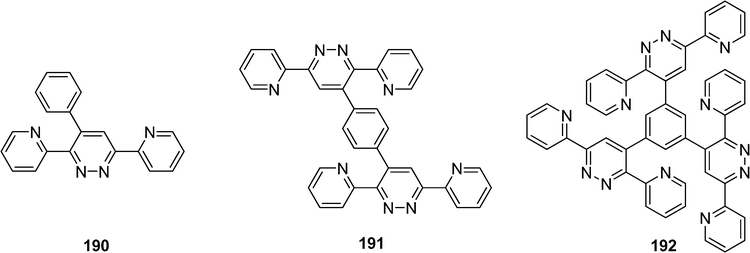
Varughese et al. have studied a series of mono-1,4-di and 1,3,5-tri-4-substituted 3,6-di(2-pyridyl)pyridazines to evaluate the substituent and steric effects in the crystal packing (Scheme 41).96 The three-dimensional architecture of the resulting assemblies was dependant of the substitution and the recognition pattern. While the asymmetric molecule 190 yielded a centrosymmetric close-packed structure, the compound 191 led to the formation of a porous assembly and the derivative 192 yielded a host–guest lattice.
 | ||
| Scheme 41 | ||
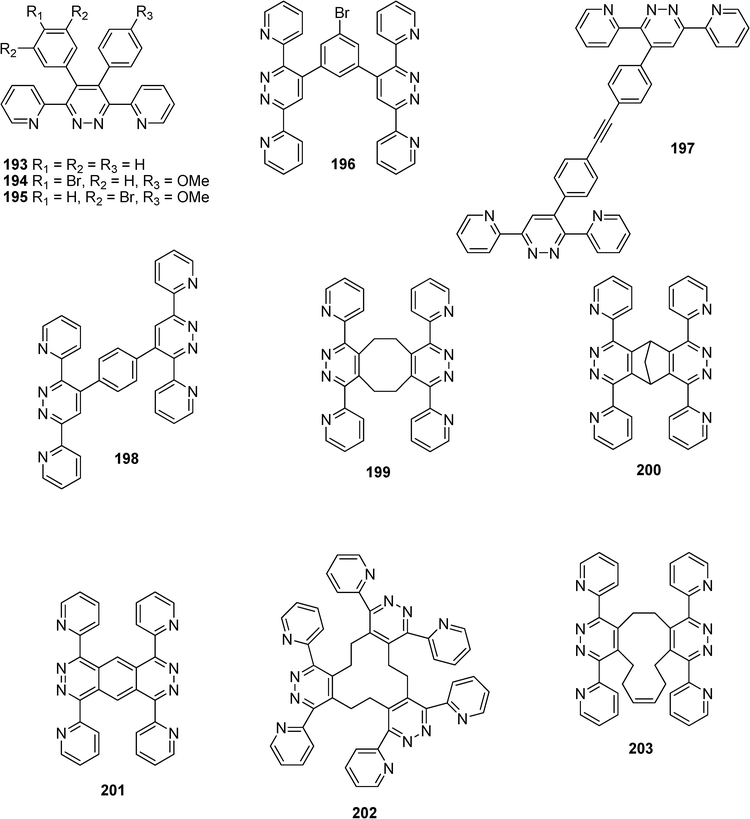
A wide range of 4-substituted and 4,5-disubstituted 3,6-di(2-pyridyl)pyridazines (193–203) have been synthesized by Constable et al. and by Thebault and coworkers as potential metal-coordinating ligands for the elaboration of supramolecular multimetallic arrays (Scheme 42).97
 | ||
| Scheme 42 | ||
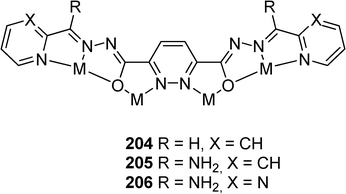
Ligands based on 3,6-pyridazinedihydrazone (tetratopic) cores with tridentates coordination pockets are highly specific and lead to efficient self-assembly with metal cations into ordered [n x n] nanoscale arrangements (Scheme 43).98 Square Mn16 [4 × 4] grids have been produced with the pyridazine ligands 204–206. A complete Cu16 [4 × 4] grid is observed with ligand 205 (Fig. 13)99 whereas an incomplete Cu12 grid observed with ligand 206.100
 | ||
| Scheme 43 | ||
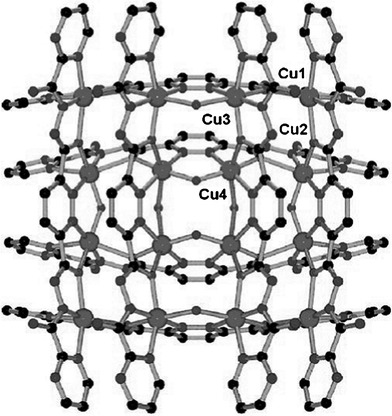
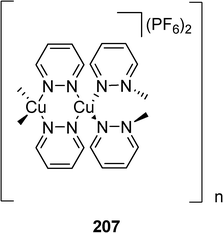
A coordination polymer incorporating pyridazine units and Cu(I) cations has been described by Plasseraud et al. (Scheme 44).102 The one-dimensional coordination polymer 207 exhibits as outstanding feature the rare structure of a meso-helix (Fig. 14).
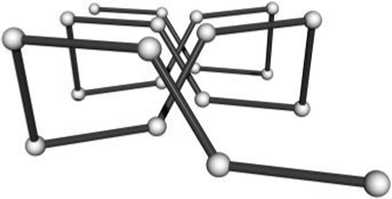 | ||
| Fig. 14 Ref. 103. Schematic presentation of the location of the copper(I) centres (balls) of coordination polymer 207. View along the crystallographic c axis. In order to clarify the meso-helical arrangement, the positions of the copper centres were scaled along c by a factor of 0.125. | ||
 | ||
| Scheme 44 | ||
The nitrogen donor ligand 4,4′-bipyridazine (4,4′-bpdz) has been used by Dommasevitch and coworkers.104 This ligand with two pairs of non-equivalent donor-N atoms can support the integrity of the coordination patterns either as bi-, tri or tetra-connected module (Fig. 15). With the silver(I) ion, unusual 3D coordination frameworks are observed involving characteristic polynuclear and polymeric silver(I)–pyridazine motifs and multiple coordination of the ligands. Incorporation of the 4,4′-bdpz ligand either as bi-, tri- or tetradentate connector with Cu(I), Cu(II) and Zn(II) metal ions has been studied.105 Various network solids have been obtained such as 1D coordination polymers Cu2(bdpz)(CH3CO2)4.4H2O and Zn(bdpz)(NO3)2, “ladder-like” polymer Cu2(bdpz)3(CF3CO2)4 as well as 3D bimodal net with [Cu3(bdpz)6(H2O)4](BF4)6·6H2O.
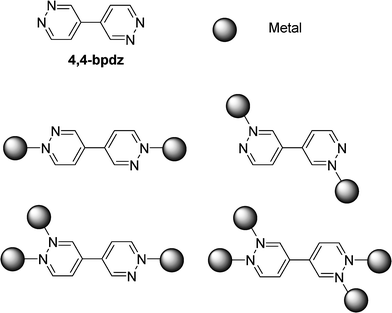 | ||
| Fig. 15 Coordination patterns of 4,4-bpdz either as bi-, tri or tetraconnected module. | ||
Various supramolecular complexes formed between alkoxyanisyl-tethered Ru(II)-tris(bipyridazine) complexes with the electron acceptor bipyridinium cyclophane (BVX4+) have been elaborated by Kropf et al. to obtain a dynamic photoinduced electron transfer system (Scheme 45).106 Effective intramolecular electron-transfer quenching proceeds in the resulting supramolecular assemblies.
Using inverse electron demand Diels–Alder reaction with 3,6-dimethyl-1,2,4,5-tetrazine dicarboxylate, the group of Julius Rebek Jr have synthesized small libraries (211–215) of low molecular weight α-helix mimetics that could be targeted to certain protein/protein interactions. These compounds have a pyridazine ring in the central position, a hydrophobic surface for recognition and a “wet edge” rich in hydrogen bond donors and acceptors to enhance solubility (Scheme 46).108
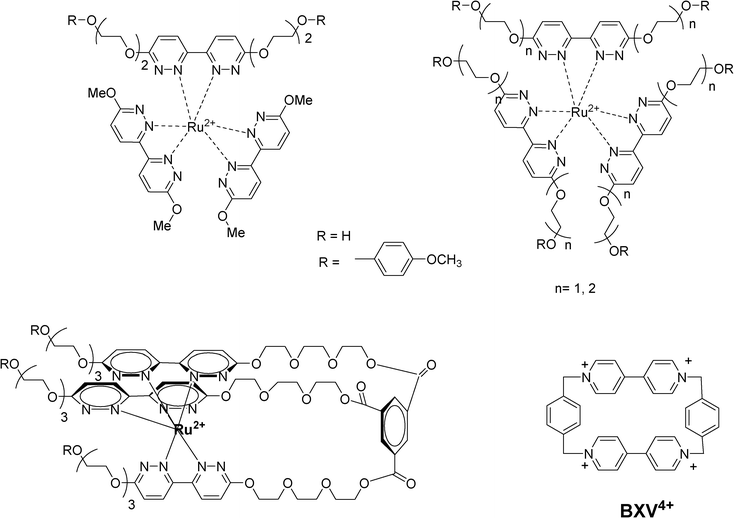 | ||
| Scheme 45 | ||
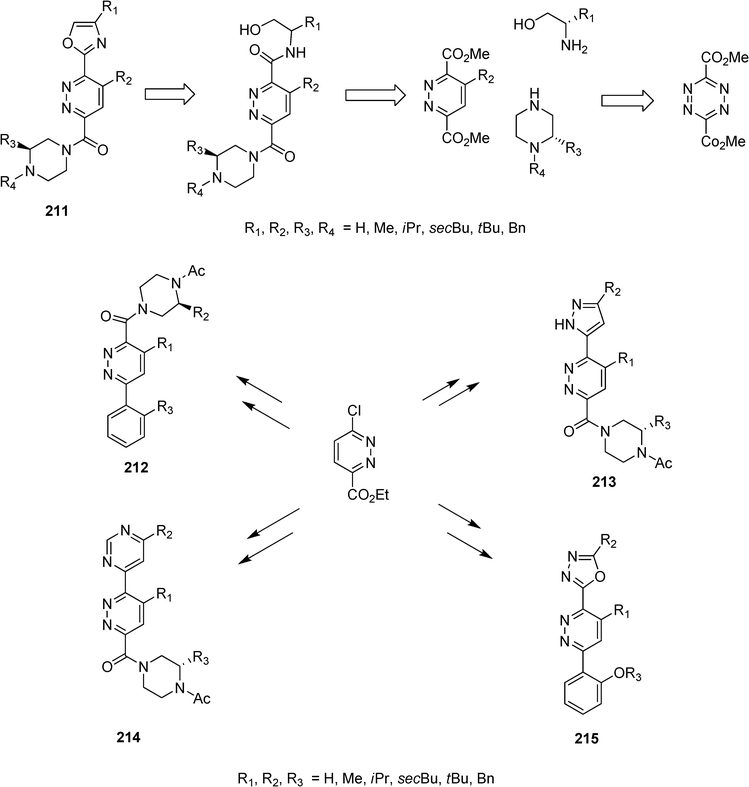 | ||
| Scheme 46 | ||
Rodriguez-Molina and coworkers have synthesized and studied the rotational dynamics of crystalline molecular compasses with pyridazine (216) and pyridazine N-oxide (217) rotators linked axially to two bulky triphenyl methyl groups by at 1,4-position with triple bonds (Scheme 47).109
 | ||
| Scheme 47 | ||
7. Conclusion and outlook
The research efforts in the field of synthesis and use of organic materials with π-conjugated scaffolds have strongly increased within the last few years. The considerable interest for these compounds is due to their applications in a wide range of electronic and optoelectronic devices. They can be used for numerous applications in various fields such as fluorescent chromophores, components of organic light-emitting devices (OLEDs) for display and lighting, field-effect transistors (FETs), single molecular electronics and non-linear optical (NLO) materials, liquid crystals and supramolecular assemblies.Incorporation of a π-deficient N-heterocycle such as pyridazine in the center of the backbone of such molecules leads to significant enhancement of their physical properties due to the π-deficient character of the pyridazine ring, used as a dipolar moiety, which favors the electron transfer. Moreover, the presence of nitrogen atoms with free electron pairs allows to the pyridazine ring to act like as effective and stable complexing agents for transition metal ions and to be used in supramolecular chemistry for the construction of highly sophisticated architectures.
The π-conjugated molecules, because of their numerous advantages such as ease of synthesis, good specificity and tunability of their photophysical properties, especially their emission wavelengths by chemical modification, have been extensively studied and developed. This review reports the recent progress in research on photoluminescent materials, liquid crystals and supramolecular assemblies which have known a high demand in material science and biological science applications.
The fast development of frequency-agile pulsed lasers has permitted the increasing attention of organic nonlinear optical (NLO) materials owing to their broad applications in the area of electronics and photonics. Among many specific phenomena derived from NLO compounds, molecular two-absorption (TPA) is gaining increasing interest for its promising applications in the field of material science and biology such as fluorescence microscopy, three dimensional optical data storage, microfabrication, high three-dimensional imaging of biological systems and photodynamic therapy.
The electron and energy transfer in donor–acceptor systems with conjugated systems have received a lot of attention due to their use as molecule based solar cells or molecular scale electronics such as for example molecular wires.
Synthesis of complex molecular architectures that could exhibit new properties and potential applications in nanotechnology is of great importance and has attracted considerable interest during the past few years. Supramolecular chemistry, with the development of self-assembly processes has strongly increases to achieve new supramolecular structures, however if the self-assembly of organic components has been a traditional area, the synthesis of supramolecular entities from organic ligands and metal ions constitutes nowadays a very efficient way. The highly sophisticated architectures obtained could be used as sensors in electronic devices, in catalysis or as novel materials with “intelligent” and tunable properties.
This wide range of conjugated structures with a pyridazine ring included in their backbone and with their numerous applications in various fields has focused an intensive worldwide effort on the design and the synthesis of such organic materials and remains always actual targets.
Among the various diazines, the pyridazine ring presents some particularities which have been used to elaborate structures with specific applications. In the pyridazine ring, presence of the two nitrogen atoms on the neighboring positions involves the strongest dipole moment (μ = 4.22D), moreover despite that diazines are soft bases favouring coordination to soft acids and the effects of back-bonding, the pyridazine unit with a pKa = 2.3 is the most basic of the diazines.
These characteristics allow pyridazines, when they are associated with pyridines (pKa = 5.2) to behave as exo-bidentate ligands, able to give oligomeric complexes and polymeric species, usually consisting of linear chains. So 3,6-di(2-pyridyl)pyridazines have been used in the construction of large and well-defined supramolecular architectures in grid-like metal complexes with applications in nanotechnology. It could be noted that besides systems such as 2,2′-bipyridines or 2,2′,6′2′′-terpyridines bis-dipyridazine ligands are of special interest due their ability to complex metallic centers with higher photostabilities toward metal–ligand photodissociation, compared to bipyridine analogues.
Advances in liquid crystals technology have triggered a strong interest in liquid crystals with negative dielectric anisotropy (Δε) which is achieved with the dipole moment perpendicular to the long molecular axis. Thanks to its high dipole moment perpendicular of 3,6 axis of the pyridazine ring, incorporation of a such disubstituted pyridazines is of great interest.
References
- (a) G. B. Barlin, ( 1982). In Chemistry of Heterocyclic Compounds, Vol. 41. New York: John Wiley and Sons Search PubMed; (b) I. I. Mangalagiu, Curr. Org. Synth., 2011, 15, 730–753 CAS.
- (a) J. Brown, ( 1962). In Chemistry of Heterocyclic Compounds, Vol. 16. New York: John Wiley and Sons Search PubMed.
- R. N. Castle, ( 1962). In Chemistry of Heterocyclic Compounds, Vol. 23. New York: John Wiley and Sons Search PubMed.
- (a) R. Grote, Y. Chen, A. Zeeck, Z. X. Chen, H. Zahner, P. Mischnick-Lubbecke and W. A. Konig, J. Antibiot., 1988, 41, 595–601 Search PubMed; (b) K. Moromoto, N. Shimada, H. Naganawa, T. Takita and H. Umezawa, J. Antibiot., 1981, 34, 1615–1618 Search PubMed; (c) T. Shiroza, N. Ebisawa, K. Furihata, T. Endo, H. Seto and N. Otake, Agric. Biol. Chem., 1982, 46, 865–867 Search PubMed.
- (a) T. Jojima, K. Oyamada and S. Tamura, Agric. Biol. Chem., 1968, 32, 1376–1381 Search PubMed; (b) S. Tamura and T. Jojima, Agr. Biol. Chem., 1963, 27, 728–733 Search PubMed; (c) S. Tamura and T. Jojima, Agr. Biol. Chem., 1963, 27, 653–729 Search PubMed.
- See for example: (a) H. Frank and G. Heinisch, In Pharmacologically Active Pyridazine Part 1, Progress in Medicinal Chemistry; Ed. Elsevier Science B. V.; Amsterdam 1990, 271 Search PubMed; (b) H. Frank and G. Heinisch, In Pharmacologically Active Pyridazine Part 2, Progress in Medicinal Chemistry; Ed. Elsevier Science B. V.; Amsterdam 1992, 141 Search PubMed; (c) J.-J. Bourguignon, S. Oumouch and M. Schmitt, Curr. Org. Chem., 2006, 10, 277–295 Search PubMed; (d) J.-M. Contreras, Y. M. Rival, S. Chayer, J.-J. Bourguignon and C.-G. Wermuth, J. Med. Chem., 1999, 42, 730–741 Search PubMed; (e) C. G. Wermuth, G. Schlewer, J.-J. Bourguignon, G. Maghioros, M. J. Bouchet, C. Moire, J. P. Kan, P. Worms and K. Biziere, J. Med. Chem., 1989, 32, 528–537 Search PubMed; (f) M. E. Castro, E. Rosa, J. A. Osuna, T. Garcia Ferreiro, M. Loza, M. I. Cadavid, J. A. Fontenla, C. F. Masaguer, J. Cid, E. Ravina, G. Garciamera, J. Rodriguez and M. L. Deceballos, Eur. J; Org. Chem., 1994, 29, 831–839 Search PubMed.
- (a) A. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897–1091 Search PubMed; (b) Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868–5923 Search PubMed; (c) H. B. Weber, J. Reichert, F. Weigend, R. Ochs, D. Beckmann, M. Mayor, R. Ahlrichs and H. von Lohneysen, Chem. Phys., 2002, 281, 113–125 Search PubMed.
- (a) C. Brulé, J.-P. Bouillon, E. Nicolaï and C. Portella, Synthesis, 2003, 436–442 Search PubMed; (b) M.-S. Lee, E.-S. Kim, A. Moon and M. S. Park, Bull. Korean Chem. Soc., 2009, 30, 83–91 Search PubMed; (c) R. Sun, Y. Zhang, F. Bi and Q. Wang, J. Agric. Food Chem., 2009, 57, 6356–6361 Search PubMed; (d) F. W. Lichtenhaler, A. Brust and E. Cuny, Green Chem., 2001, 3, 201–209 Search PubMed.
- (a) M. Cecchi, A. Micoll and D. Giomi, Tetrahedron, 2006, 62, 11281–12287 Search PubMed; (b) M. S. Shin, Y. J. Kang, H. A. Chung, J. W. Park, D. H. Kweon, W. S. Lee, Y. J. Yoon and S. K. Kim, J. Heterocycl. Chem., 1999, 36, 1135–1142 Search PubMed; (c) P. Coad, R. A. Coad, S. Clough, J. Hyepock, R. Salisbury and C. Wilkins, J. Org. Chem., 1963, 28, 218–221 Search PubMed; (d) P. Coad and R. A. Coad, J. Org. Chem., 1963, 28, 1919–1921 Search PubMed; (e) P. Coad, R. A. Coad and J. Hyepock, J. Org. Chem., 1964, 29, 1751–1754 Search PubMed.
- (a) A. Turck, N. Plé, F. Mongin and G. Quéguiner, Tetrahedron, 2001, 57, 4489–4505 Search PubMed; (b) F. Chevallier and F. Mongin, Chem. Soc. Rev., 2008, 37, 595–609 Search PubMed.
- (a) S. Nara, J. Martinez, C. G. Wermuth and I. Parrot, Synlett, 2006, 19, 3185–3204 Search PubMed; (b) B. U. W. Maes, P. Tapolcsányi, C. Meyers and P. Mátyus, Curr. Org. Chem., 2006, 10, 377–417 Search PubMed; (c) B. U. W. Maes, J. Kosmrly and G. L. F. Lemier, J. Heterocycl. Chem., 2002, 39, 535–543 Search PubMed.
- A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 Search PubMed.
- (a) C. Fruit, A. Turck, N. Plé, L. Mojovic and G. Quéguiner, Tetrahedron, 2002, 58, 2743–2753 Search PubMed; (b) A. Turck, A. N. Plé, A. Lepretre-Gaquere and G. Quéguiner, Heterocycles, 1998, 49, 205–214 Search PubMed; (c) M. D. Helm, J. E. Moore, A. Plant and J. P. A. Harrity, Angew. Chem., Int. Ed., 2005, 44, 3889–3892 Search PubMed.
- (a) J.-J. Vanden Eynde, L. Pascal, Y. Van Haverbeke and P. Dubois, Synth. Commun., 2001, 31, 3167–3173 Search PubMed; (b) S. P. Gorugantula, G. M. Carrero-Martinez, S. W. Dantale and B. C. G. Soderberg, Tetrahedron, 2010, 66, 1800–1805 Search PubMed; (c) K. Ohno, K. Arima, S. Tanaka, T. Yamagata, H. Tsurugi and K. Mashima, Organometallics, 2009, 28, 3256–3263 Search PubMed.
- (a) S. Seminario and J. M. Tour, In Molecular Electronics-Science and Technology; Aviran, A.; Ratner, M., ed.; New York Academy of Science: New York, 1998 Search PubMed; (b) K. Müllen and G. Wegner, ed. In Electronics Materials: The Oligomer Approach; Wiley-VCH: New York, 1998 Search PubMed; (c) Molecular Materials in Electronic and Optoelectronic Devices , Special Issue: J. R. Sheats and P. F. Barbara, ed. Acc. Chem. Res. 1999, 32 Search PubMed; (d) J. M. Tour, Acc. Chem. Res., 2000, 33, 791–804 Search PubMed; (e) K. Müllen, U. Scherf, ed.Organic Light Emitting Devices, Wiley-VCH, Weinheim, 2006 Search PubMed; (f) T. A. Skotheim and J. R. Reynolds, ed. Handbook of Conducting Polymers, 3rd Ed., CRC Press, Boca Raton, 2006 Search PubMed.
- U. Scherf, Top. Curr. Chem., 1999, 201, 163–222 Search PubMed.
- H. Meier, T. Lifka, P. Seus, A. Oehlhof and S. Hillmann, Tetrahedron, 2008, 64, 10754–10760 Search PubMed.
- D. Dorohoi, H. Partenie, L. Chiran and C. Anton, J. Chem. Phys. Phys. Chem. Biol., 1994, 91, 419–431 Search PubMed.
- H. H. Perkampu, T. Bluhm and J. V. Knop, Spectrochim. Acta, Part A, 1972, 28, 2163–2177 Search PubMed.
- S. A. Haroutounian and J. A. Katzenellenbogen, Tetrahedron, 1995, 51, 1585–1598 Search PubMed.
- V. Schmitt, S. Glang, J. Preis and H. Detert, Sens. Lett., 2008, 6, 1–7 Search PubMed.
- C. Hadad, C. Fiol-Petit, A.-S. Cornec, G. Dupas, Y. Ramondenc and N. Plé, Heterocycles, 2010, 81, 1445–1457 Search PubMed.
- T. Yasuda, Y. Sakai, S. Aramaki and T. Yamamoto, Chem. Mater., 2005, 17, 6060–6068 Search PubMed.
- J. Do, J. Huh and E. Kim, Langmuir, 2009, 25, 9405–9412 Search PubMed.
- J. Do, Y. Kim, A.-J. Attias, D. Kreher and E. Kim, J. Nanosci. Nanotechnol., 2010, 10, 6874–6878 Search PubMed.
- F. Lincker, D. Kreher, A.-J. Attias, J. Do, E. Kim, P. Hapiot, N. Lemaìtre, B. Geffroy, G. Ulrich and R. Ziessel, Inorg. Chem., 2010, 49, 3991–4001 Search PubMed.
- B. R. Kim, S.-D. Cho, H.-G. Lee, H.-S Yim, M.-J. Kim, J. Hwang, S. E. Park, J.-J. Kim, K.-J. Jung and Y.-J. Yoon, J. Heterocycl. Chem., 2009, 46, 691–701 Search PubMed.
- M. Vasilescu, R. Bandula, O. Cramariuc, T. Hukka, H. Lemmetyinen, T. T. Rantala and F. Dumitrascu, J. Photochem. Photobiol., A, 2008, 194, 308–317 Search PubMed.
- K. M. K. Swamy, M. S. Park, S. J. Han, S. K. Kim, J. H. Kim, C. Lee, H. Bang, Y. Kim, S.-J. Kim and J. Yoon, Tetrahedron, 2005, 61, 10227–10234 Search PubMed.
- (a) G. N. Zbancioc, T. Huhn, U. Groth, C. Deleanu and I. I. Mangalagiu, Tetrahedron, 2010, 66, 4298–4306 Search PubMed; (b) G. Zbancioc and I. I. Mangalagiu, Tetrahedron, 2010, 66, 278–282 Search PubMed.
- Z. Q. Gao, B. X. Mi, H. L. Tam, K. W. Cheah, C. H. Chen, M. S. Wong, S. T. Lee and C. S. Lee, Adv. Mater., 2008, 20, 774–778 Search PubMed.
- Reprinted with permission from ref. 31. Copyright 2008, from John Wiley and sons.
- B. X. Mi, P. F. Wang, Z. Q. Gao, C. S. Lee, S. T. Lee, X. M. Hong, M. S. Wong, P. F. Xia, K. W. Cheah, C. H. Chen and W. Huang, Adv. Mater., 2009, 21, 339–343 Search PubMed.
- Y. Fang, Y. Li, S. Wang, Y. Meng, J. Peng and B. Wang, Synth. Met., 2010, 160, 2231–2238 Search PubMed.
- Q. Yu, A. S. Zhang, T.-L. Hu and X.-H. Bu, Solid State Sci., 2010, 12, 1484–1489 Search PubMed.
- M. Panigati, D. Donghi, G. D'Alfonso, P. Mercandelli, A. Sironi and L. D'Alfonso, Inorg. Chem., 2006, 45, 10909–10921 Search PubMed.
- D. Donghi, G. D'Alfonso, M. Mauro, M. Panigati, P. Mercandelli, A. Sironi, P. Mussini and L. D'Alfonso, Inorg. Chem., 2008, 47, 4243–4255 Search PubMed.
- (a) D. Asil, A. Cihaner and A. M. Önal, Electrochim. Acta, 2009, 54, 6740–6746 Search PubMed; (b) N. Atilgan, F. Algi, A. M. Önal and A. Cihaner, Tetrahedron, 2009, 65, 5776–5781 Search PubMed.
- Reprinted with permission from ref. 38a. Copyright 2009, from Elsevier.
- D. Asil, A. Cihaner and M. Önal, Chem. Commun., 2009, 307–309 Search PubMed.
- (a) J. R. Heath and M. A. Ratner, Phys. Today, 2003, 56, 43 Search PubMed and references therein.; (b) T. C. Lin, S. J. Chung, K. S. Kim, X. P. Wang, G. S. He, J. Swiatkiewicz, H. E. Pudavar, P. N. Prasad In Polymers for Photonics Applications II (Ed.: K.-S. Lee), Springer, Berlin, 2003, pp. 157 –193 Search PubMed; (c) P. N. Prasad and B. A. Reinhardt, Chem. Mater., 1990, 2, 660–669 Search PubMed; (d) M. Blanchard-Desce, C. R. Phys., 2002, 3, 439–448 Search PubMed.
- (a) W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73–76 Search PubMed; (b) C. Xu, W. Zipfel, J. B. Shear, R. M.; Williams and W. W. Webb, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 10763–10768 Search PubMed.
- (a) S. Maruo, O. Nakamura and S. Kawata, Opt. Lett., 1997, 22, 132–134 Search PubMed; (b) B. H. Cumpston, S. P. Ananthavel, S. Barlow, D. L. Dyer, J. E. Ehrlich, L. L. Erskine, A. A. Heikal, S. M. Kuebler, I.-Y. S. Lee, D. McCord-Maughon, J. Qin, H. Roëckel, M. Rumi, X. L. Wu, S. R. Marder and J. W. Perry, Nature, 1999, 398, 51–54 Search PubMed; (c) S. Kawata, H. B. Sun, T. Tanaka and K. Takada, Nature, 2001, 412, 697–698 Search PubMed; (d) W. Zhou, S. M. Kuebler, K. L. Braun, T. Yu, J. K. Cammack, C. K. Ober, J. W. Perry and S. R. Marder, Science, 2002, 296, 1106–1109 Search PubMed.
- (a) D. A. Parthenopoulos and P. M. Rentzepis, Science, 1989, 245, 843–845 Search PubMed; (b) J. H. Strickler and W. W. Webb, Opt. Lett., 1991, 16, 1780–1782 Search PubMed; (c) A. S. Dvornikov and P. M. Rentzepis, Opt. Commun., 1995, 119, 341–346 Search PubMed; (d) K. D. Belfield and K. J. Schafer, Chem. Mater., 2002, 14, 3656–3662 Search PubMed; (e) K. D. Belfield, Y. Liu, R. A. Negres, M. Fan, G. Pan, D. J. Hagan and F. E. Hernandez, Chem. Mater., 2002, 14, 3663–3667 Search PubMed.
- (a) G. S. He, G. C. Xu, P. N. Prasad, B. A. Reinhardt, J. C. Bhatt, R. McKellar and A. G. Dillard, Opt. Lett., 1995, 20, 435–437 Search PubMed; (b) J. E. Ehrlich, X. L. Wu, I. Y. S. Lee, Z. Y. Hu, H. Röckel, S. R. Marder and J. W. Perry, Opt. Lett., 1997, 22, 1843–1845 Search PubMed.
- (a) J. R. Starkey, A. K. Rebane, M. A. Drobizhev, F. Meng, A. Gong, A. Elliott, K. McInnerney and C. W. Spangler, Clin. Cancer Res., 2008, 14, 6564–6573 Search PubMed; (b) H. A. Collins, M. Khurana, E. H. Moriyama, A. Mariampillai, E. Dahlstedt, M. Balaz, M. K. Kuimova, M. Drobizhev, V. X. D. Yang, D. Phillips, A. Rebane, B. C. Wilson and H. L. Anderson, Nat. Photonics, 2008, 2, 420–424 Search PubMed; (c) K. Ogawa and Y. Kobuke, Anti-Cancer Agents Med. Chem., 2008, 8, 269–279 Search PubMed.
- S. Achelle and N. Plé, Curr. Org. Synth. 2011 Search PubMed, accepted.
- P. H. Huang, J.-Y. Shen, S.-C. Pu, Y.-S. Wen, J. T. Lin, P. T. Chou and M.-C. P. Yeh, J. Mater. Chem., 2006, 16, 850–857 Search PubMed.
- T. Yamamoto, A. Kumagai, K. Saito and T. Nagai, J. Nanosci. Nanotechnol., 2009, 9, 670–672 Search PubMed.
- T.-R. Cheng, C.-H. Huang, L.-B. Gan, C.-P. Luo, A.-C. Yu and X.-S. Zhao, J. Mater. Chem., 1998, 8, 931–935 Search PubMed.
- F. Ma, Z.-R. Li, H.-L. Xu, Z.-J. Li, Z.-S. Li, Y. Aoki and F. L. Gu, J. Phys. Chem. A, 2008, 112, 11462–11467 Search PubMed.
- B. Albinson, M. P. Eng, K. Petterson and M. U. Winters, Phys. Chem. Chem. Phys., 2007, 5847–5864 Search PubMed.
- (a) S. Forrest, Nature, 2004, 428, 911–918 Search PubMed; (b) M. Mas-Torrent and C. Rovira, Chem. Soc. Rev., 2008, 37, 827–838 Search PubMed.
- (a) Y. Kunigi, K. Takimiya, N. Negishi, T. Otsubo and Y. Aso, J. Mater. Chem., 2004, 14, 2840–2841 Search PubMed; (b) R. J. Chesterfield, C. R. Newman, T. M. Pappenfls, P. C. Ewbank, M. H. Haukaas, K. R. Mann, L. L. Miller and C. D. Frisbie, Adv. Mater., 2003, 15, 1278–1282 Search PubMed.
- (a) Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868–5923 Search PubMed; (b) S. Günes, H. Neugebauer and N. S. Saricifttci, Chem. Rev., 2007, 107, 1324–1338 Search PubMed.
- (a) M. Kropf, E. Joselevich, H. Dürr and I. Willner, J. Am. Chem. Soc., 1996, 118, 655–665 Search PubMed; (b) M. Kropf, D. van Loyen, O. Schwarz and H. Dürr, J. Phys. Chem. A, 1998, 102, 5499–5505 Search PubMed.
- O. Schwarz, D. van Loyen, S. Jockusch, N. J. Turro and H. Dürr, J. Photochem. Photobiol., A, 2000, 132, 91–98 Search PubMed.
- Reprinted with permission from ref. 57. Copyright 2000, from Elsevier.
- D. Gendron, P.-O. Morin, A. Najari and M. Leclerc, Macromol. Rapid Commun., 2010, 31, 1090–1094 Search PubMed.
- Y. Itoh, H. Seki, T. Uchida and Y. Masuda, Jpn. J. Appl. Phys., 1991, 1296–1699 Search PubMed.
- F. Gharadjedaghi, Mol. Cryst. Liq. Cryst., 1981, 68, 127–125 Search PubMed.
- (a) F. Guittard and S. Géribaldi, In Anisotropic Organic Materials, ACS Symposium series. 2001, Vol 798, pp. 180-194 Search PubMed; (b) D. Guillon, M. Z. Cherkaoui, P. Sebastião, S. Méry, J. F. Nicoud, Y. Galerne, In Anisotropic Organic Materials, ACS Symposium series. 2001, Vol 798, pp. 214-226 Search PubMed; (c) E. F. Carr, in Ordered Fluids and Liquid Crystals. 1967, Vol. 63, pp 76-88 Search PubMed.
- M. E. Glendenning, J. W. Goodby, M. Hird and K. J. Toyne, J. Chem. Soc., Perkin Trans. 2, 2000, 27–34 Search PubMed.
- (a) M. Schadt, M. Petrzilka, P. R. Gerber, A. Villiger and G. Trickes, Mol. Cryst. Liq. Cryst., 1983, 94, 139–153 Search PubMed; (b) V. Reiffenrath, J. Krause, H. J. Plach and G. Weber, Liq. Cryst., 1989, 5, 159–170 Search PubMed.
- R. Paschke, U. Rosenfild and H. Zaschke, Liq. Cryst., 1992, 11, 145–150 Search PubMed.
- S. Achelle, N. Plé, D. Kreher, F. Mathevet, A. Turck and A.-J. Attias, Heterocycles, 2008, 75, 357–374 Search PubMed.
- S. Achelle, N. Plé, A. Turck, J.-P. Bouillon and C. Portella, J. Heterocycl. Chem., 2006, 43, 1243–1249 Search PubMed.
- J. H. Wild, K. Bartle, N. T. Kirkman, S. M. Kelly, M. O'Neill, T. Stirner and R. P. Tuffin, Chem. Mater., 2005, 17, 6354–6360 Search PubMed.
- T. Lifka, G. Zerban, P. Seus, A. Oehlhof and H. Meier, Tetrahedron, 2008, 64, 6551–6560 Search PubMed.
- T. Lifka, P. Seus, A. Oehlhof and H. Meier, Helv. Chim. Acta, 2009, 92, 281–290 Search PubMed.
- Y. S. Park, D. Kim, H. Lee and B. Moon, Org. Lett., 2006, 8, 4699–4702 Search PubMed.
- Reprinted with permission from ref. 71. Copyright 2006, from American Chemical Society.
- S. Senthil, N. Poomathi and P. Kannan, J. Appl. Polym. Sci., 2007, 103, 3924–3930 Search PubMed.
- J. W. Slater, D. P. Lydon and J. P. Rouske, J. Organomet. Chem., 2002, 645, 246–255 Search PubMed.
- M. M. Teoh, S. L. Liu and T. S. Chung, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 2230–2242 Search PubMed.
- L. Carlucci, G. Ciani, D. M. Proserpio and A. Sironi, Inorg. Chem., 1998, 37, 5941–5943 Search PubMed.
- Reprinted with permission from ref. 76. Copyright 1998, from American Chemical Society.
- (a) L. A. Cuccia, J.-C. Homo, J.-M. Lehn and M. Schmutz, Angew. Chem., Int. Ed., 2000, 39, 233–237 Search PubMed; (b) L. A. Cuccia, E. Riuz, J.-M. Lehn, J.-C. Homo and M. Schmutz, Chem.–Eur. J., 2002, 8, 3448–3457 Search PubMed.
- Reprinted with permission from ref. 78b. Copyright 2002, from John Wiley and sons.
- M.-T. Youinou, N. Rahmouni, J. Fischer and J. A. Osborn, Angew. Chem., Int. Ed. Engl., 1992, 31, 733–735 Search PubMed.
- (a) P. N. W. Baxter, J.-M. Lehn, B. O. Kneisel and D. Fenske, Angew. Chem., Int. Ed. Engl., 1997, 36, 1978–1981 Search PubMed; (b) B. L. Schottel, H. T. Chifotides, M. Shatruk, A. Chouai, L. Pérez, J. Bacsa and K. R. Dunbar, J. Am. Chem. Soc., 2006, 128, 5895–5912 Search PubMed.
- N. D. Sung, K.-S. Yun, T.-Y. Kim, K.-Y. Choi, M. Suh, J.-G. Kim, I.-K. Suh and J. Chin, Inorg. Chem. Commun., 2001, 4, 377–380 Search PubMed.
- I. Weissbuch, P. N. W. Baxter, I. Kuzmenko, H. Cohen, S. Cohen, K. Kjaer, P. B. Howes, J. Als-Nielsen, J.-M. Lehn, L. Leiserowitz and M. Lahav, Chem.–Eur. J., 2000, 6, 725–732 Search PubMed.
- Reprinted with permission from ref. 83. Copyright 2000, from John Wiley and Sons.
- R. Hoogenboom, G. Kickelbick and U. S. Schubert, Eur. J. Org. Chem., 2003, 4887–4896 Search PubMed.
- E. C. Constable, C. E. Housecroft, M. Neuburger, S. Reymann and S. Schaffner, Eur. J. Inorg. Chem., 2008, 3540–3548 Search PubMed.
- E. C. Constable, C. E. Housecroft, M. Neuburger, S. Reymann and S. Schaffner, Chem. Commun., 2004, 1056–1057 Search PubMed.
- E. C. Constable, C. E. Housecroft, M. Neuburger, S. Reymann and S. Schaffner, Eur. J. Inorg. Chem., 2008, 3540–3548 Search PubMed.
- Reprinted with permission from ref. 88. Copyright 2008, from John Wiley and sons.
- R. Hoogenboom, B. C. Moore and U. S. Schubert, Chem. Commun., 2006, 4010–4012 Search PubMed.
- R. Hoogenboom, D. Wouters and U. S. Schubert, Macromolecules, 2003, 36, 4743–4749 Search PubMed.
- R. Hoogenboom, B. C. Moore and U. S. Schubert, Macromol. Rapid Commun., 2010, 31, 840–845 Search PubMed.
- B. R. Manzano, F. A. Jalón, I. M. Ortiz, M. L. Soriano, F. Gómez de la Torre, J. Elguero, M. A. Maestro, K. Mereiter and T. D. W. Claridge, Inorg. Chem., 2008, 47, 413–428 Search PubMed.
- (a) W.-W. Sun, Q. Yue, A.-L. Cheng and E. Q. Gao, Cryst. Eng. Commun., 2008, 10, 1384–1394 Search PubMed; (b) W.-W. Sun, A.-L. Cheng, Q.-X. Jia and E.-Q. Gao, Inorg. Chem., 2007, 46, 5471–5473 Search PubMed.
- S. Sobanska, J.-P. Wignacourt, P. Conflant, M. Drache, M. Legrenée and E. M. Holt, New J. Chem., 1999, 23, 393–396 Search PubMed.
- S. Varughese, G. Cooke and S. M. Draper, Cryst. Eng. Commun., 2009, 11, 1505–1508 Search PubMed.
- (a) E. C. Constable, C. E. Housecroft, M. Neuburger, S. Reymann and S. Schaffner, Eur. J. Org. Chem., 2008, 1597–1607 Search PubMed; (b) F. Thébault, A. J. Blake, C. Wilson, N. R. Champness and M. Schöder, New J. Chem., 2006, 30, 1498–1508 Search PubMed.
- (a) K. V. Shuvaev, T. S. M. Abedin, C. A. McClary, L. N. Dawe, J. L. Collins and L. K. Thomson, Dalton Trans., 2009, 2926–2939 Search PubMed; (b) S. K. Dey, T. S. M. Abedin, L. N. Dawe, S. S. Tandon, J. L. Collins, L. K. Thompson, A. V. Postnikov, M. S. Alam and P. Müller, Inorg. Chem., 2007, 46, 7767–7781 Search PubMed; (c) S. K. Dey, L. K. Thompson and L. N. Dawe, Chem. Commun., 2006, 4967–4969 Search PubMed.
- L. N. Dawe and L. K. Thompson, Angew. Chem., Int. Ed., 2007, 46, 7440–7444 Search PubMed.
- C. J. Matthews, S. T. Onions, G. Morata, M. Bosch Salvia, M. R. J. Elsegood and D. J. Price, Chem. Commun., 2003, 320–321 Search PubMed.
- Reprinted with permission from ref. 99. Copyright 2007, from John Wiley and sons.
- L. Plasseraud, H. Maid, F. Hampel and R. W. Saalfrank, Chem.–Eur. J., 2001, 7, 4007–4011 Search PubMed.
- Reprinted with permission from ref. 102. Copyright 2001, from John Wiley and sons.
- K. V. Domasevitch, P. V. Solntsev, H. A. Gural'skiy, H. Krautscheid, E. B. Rusanov, A. N. Chernega and J. A. K. Howard, Dalton Trans., 2007, 3893–3905 Search PubMed.
- K. V. Domasevitch, I. A. Gural'skiy, P. V. Solntsev, E. B. Rusanov, H. Krautscheid, J. A. K. Howard and A. N. Chernega, Dalton Trans., 2007, 3140–3148 Search PubMed.
- M. Kropf, E. Joselevich, H. Dürr and I. Willner, J. Am. Chem. Soc., 1996, 118, 655–665 Search PubMed.
- Reprinted with permission from ref. 94b. Copyright 2007, from American Chemical Society.
- (a) S. M. Biros, L. Moisan, E. Mann, A. Carella, D. Zhai, J. C. Reed and J. Rebek Jr, Bioorg. Med. Chem. Lett., 2007, 17, 4641–4645 Search PubMed; (b) A. Volonterio, L. Moisan and J. Rebek Jr, Org. Lett., 2007, 9, 3733–3736 Search PubMed.
- B. Rodriguez-Molina, M. E. Ochoa, N. Farfán, R. Santillan and M. A. García-Garibay, J. Org. Chem., 2009, 74, 8554–8565 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |

![Ref. 89. Molecular structure of the [Ag2(185)2]2+ cation in [Ag2(185)2]-[BF4]2·2.6MeCN.](/image/article/2011/RA/c1ra00207d/c1ra00207d-f10.gif)