Two unique (4,5,6)-connected 2D CdII coordination polymers based on the 5-nitro-1,2,3-benzenetricarboxylate ligand†
Lu-Fang
Ma
ab,
Jian-Hua
Qin
a,
Li-Ya
Wang
*a and
Dong-Sheng
Li
*b
aCollege of Chemistry and Chemical Engineering, Luoyang Normal University, Luoyang 471022, China. E-mail: wlya@lynu.edu.cn
bCollege of Mechanical & Material Engineering, Functional Materials Research Institute, China Three Gorges University, Yichang 443002, China. E-mail: lidongsheng1@126.com
First published on 1st August 2011
Abstract
Two new 2D coordination polymers, [Cd3(nbta)2(H2O)4] (1) and [Cd3(nbta)2(bbi)2H2O)2] (2) (H3nbta = 5-nitro-1,2,3-benzenetricarboxylate, bbi = 1,1′-(1,4-butanediyl)bis(imidazole)) have been synthesized and structurally characterized by single X-ray structure analysis. The versatile conformations and binding modes of the nbta ligand as well as the coordination environment of the CdII atoms result in two unique (4,5,6)-connected 2D CdII-coordination polymers, both of which show enthralling helical structures.
The design and synthesis of coordination polymer frameworks are of great current interest, stemming from their potential applications as functional materials as well as their structural diversity and intriguing topologies.1–4 Network topology has been proven to be a powerful tool for research of coordination polymers, not only for the importance of reducing complicated frameworks to simple node-and-linker referenced nets but also for guiding the rational design of certain functional materials with desirable properties.5,6 To date, a variety of network topologies have been realized by real crystal structures since Wells described topologies in his classic monographs on networks decades ago7, most of which are based on 3-D networks with three-, four-, or six-connected topologies, such as srs, dia, cds, nbo and pcuetc.8 Meanwhile, the simple 2D layers with relevant connectivity are usually constructed from uniform and regular polygons and their corresponding network symbols are designated as 63, 44, and 36, respectively.9 However, mixed connectivity of (4,5,6)-connected 2-D layers have rarely been reported so far.10
It's well-known that the coordination geometries of the metal centers and the coordination behaviours of the organic ligands are the main keys to achieving the target coordination polymers. Benzene-based multicarboxylic acid ligands, as good candidates for the construction of coordination polymers, have been extensively used.115-Nitro-1,2,3-benzenetricarboxylate (H3nbta) ligand, a derivative of 1,2,3-benzenetricarboxylate (H3bta),12 remains largely unexplored hitherto in the field of coordination polymers.13 However, H3nbta may provide the potential for constructing unpredictable and interesting network structures due to the existence of a non-coordinating electron-withdrawing nitro-group on the aromatic backbone, which will have a profound impact on the electron density of such a ligand and result in different physical and chemical properties.
Reaction of H3nbta and CdII acetate with or without bbi at 160 °C for 3 days under hydrothermal conditions generates complexes 1 and 2.‡ The compositions were confirmed by elemental analysis, IR spectrum and single crystal X-ray analysis.§¶ The phase purities of the bulk samples were identified by XRPD (Fig. S1 , ESI†)
Single crystal X-ray diffraction analysis (ESI†) reveals that complex 1 crystallizes in a P21/c space group, and the asymmetric unit of which contains three crystallographically independent CdII centers. As shown in Fig. S2 , ESI†, Cd1 centers a distorted square pyramid, defined by four carboxylic oxygen atoms from four different nbta ligands and one water molecule. The O3, O13, and O18 atoms are weakly coordinated to the Cd1 ion with a Cd–O distances of 2.667(2), 2.679(2), and 2.668(2) Å, respectively. As for Cd2 and Cd3, if we neglect the weak Cd3–O10 bond (2.787(2) Å), both of which have a distorted pentagonal–bipyramidal geometry coordination environment, formed by five carboxylic oxygen atoms from four different nbta ligands and two water molecules with the axial sites O1–Cd2–O11C and O6D–Cd3–O12 angles of 150.61(5) and 152.97(5)°, respectively. (see Table S1, ESI†). The nbta ligands in 1 are completely deprotonated, showing two kinds of heptadentate coordination modes (μ1–η1: η1/μ2–η1: η1/μ3–η1: η2 and μ1–η1: η0/μ1–η1: η1/μ4–η2: η2, see Fig. S3, ESI†). In such a ligating manner, the CdII centers are connected by the nbta ligands to afford a complicated 2D layered structure containing two types of helical layers (Cd2– and Cd3–nbta helical layer see Fig. 1a). For the first one, two μ2–O11 atoms bridge Cd2 atoms to form a [Cd2O2] dimer, which are then aligned by O2C7–C1–C2–C8O2 groups into a 21 helical array along the [001] axis. The adjacent right- and left-handed helices are fused to each other by sharing the [Cd2O2] dimer to generate a 2D Cd2–nbta helical layer. In the Cd3–nbta helical layer, a pair of O2C7–C1–C6–C9O2 groups link the Cd3 atoms to result in a 14-numbered ring, and the adjacent ones are arranged by O2C17–C11–C12–C18O2groups to give rise to right- and left-handed helical chains. Interestingly, two same handedness helices from different helical layer interweave to each other by the connectivity of Cd2–O1–C7–O2–Cd3 and Cd1–O to produce double-stranded right- or left-handed helices (see Fig. S4, ESI†). From the viewpoint of topology, the 4-connected Cd2 and 5-connected Cd1, Cd3 inorganic nodes as well as the 6-connected nbta organic nodes interlink into a new (4,5,6)-connected 2D net (see Fig. 1b) with the point symbol of (44.5.6)(32.45.52.6)(32.44.52.62)(3.48.52.64)(3.48.53.63) analyzed by the TOPOS program.
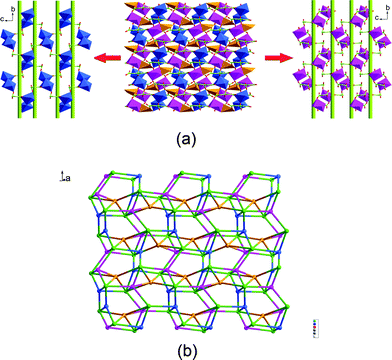 | ||
| Fig. 1 (a) The 2D layer of 1 containing two types of helical layers (Cd2– and Cd3–nbta helical layer). (b) Schematic representation of the (4,5,6)-connected (44.5.6)(32.45.52.6)(32.44.52.62)(3.48.52.64)(3.48.53.63) topology (Cd1: orange; Cd2: blue; Cd3: purple; nbta ligand: green). | ||
When the flexible bis-(imidazole) ligand bbi was taken into the above system, a distinct 2D helical layer was obtained. Complex 2 crystallizes in a P21/c space group, and the asymmetric unit has two different CdII atoms. As illustrated in Fig. S5, ESI†, each CdII atom is six coordinated but locates in different octahedral coordination geometry. Five O atoms from three nbta ligands and one N atom from the bbi molecule complete the distorted octahedral coordination sphere of Cd1 atom. While Cd2 atom is coordinated by two O atoms from two nbta ligands, two N atoms from two bbi molecules as well as two aqua ligands (see Table S1, ESI† for detailed bond lengths and angles). Different from 1, the completely deprotonated nbta in 2 acts as a hexadentate ligand. Three carboxylic groups adopt μ1–η1: η1, μ2–η0: η2 and μ2–η1: η1 bridging modes, respectively (see Fig. S6, ESI†). The bbi ligand acts as a linear bidentate ligand. Based on such bridging modes, the CdII atoms are extended by nbta and bbi ligands into a 2D layer (see Fig. 2a). The helical chains, formed by bbi ligands and O2C8–C2–C3–C9O2 groups bridging between the CdII atoms running along a crystallographic 21 axis in the [100] direction with a pitch of 7.61 Å, are observed in the 2D net. The neighbouring right- and left-handed helices are joined together by the connectivity of Cd1–C7O2. From a topological perspective, all the CdII atoms and nbta ligands can be regarded as 4-connected nodes. Thus the 2D net can be described as a trinodal 4-connected net with the Schläfli symbol of (42.53.6)(52.62.72)(42.53.6). However, if the strong hydrogen bonding between carboxylic oxygen atoms and coordinated water molecules [O(9)–H(2W)⋯O(3)a, symmetry codes: a = −x + 1, −y + 1, −z + 3; H⋯O /O⋯O distances: 2.001/2.784 Å; angle: 157.32°] is considered (see Fig. 2b), the nbta ligands become 5-connecting nodes, [Cd2(H2O)2] moieties act as the 6-connected nodes and Cd1 remaining 4-connected nodes. Accordingly, this 2D layer can also be considered as the overall (4,5,6)-connected trinodal net with the Schläfli symbol of (43.5.62)(45.53.62)(44.56.64.7) (see Fig. 2c).
 | ||
| Fig. 2 (a) 2D helical layer of 2 parallel along the ab plane. (b) The intra-layer hydrogen bonding between carboxylic oxygen atoms and coordinated water molecules (only the pertinent backbones of nbta ligand are shown). (c) Schematic representation of the 4- and (4,5,6)-connected net considering the intra-layer hydrogen bonding (Cd1: orange; Cd2: blue; nbta ligand: green). | ||
Recently, we isolated a pair of 4- and 6-connected 2D supramolecular architectures14 by the assembly of 1,3-bis(4-pyridyl)propane (bpp) linker with CoII and different isophthalate tectons. The unique network topologies can be realized by the different linkages of the adjacent 44 layers. Also, for the overall 2D net of 1, if the Cd1 nodes are ignored, it changes into a 4-connected (43.63) topology. Of the 4-connected Cd2, Cd3 and nbta nodes, three form the puckering 63 net (blue and purple in Fig. S7a, ESI†) while the interlayer bridge of the fourth are perpendicularly aligned to the net. In the same way, the (4,5,6) connected net of 2 can be regarded as two corrugated 63 nets sharing Cd2 nodes with the remaining linker locating at the interlayer (see Fig. S7b, ESI†).
As shown in Table S2, ESI† (two kinds of nbta3− ligands in 1), one outer carboxylic group of each nbta3− ligand is nearly coplanar with the corresponding aromatic rings, while the remaining outer carboxyl group has greatly deviated from the coplanar of the aromatic rings, the dihedral angles between the outer carboxylic groups and the corresponding aromatic rings are 47.2, 31.9, and 131.0°, respectively. This geometry of the nbta3− ligand plays an important role in the formation of the helical structures in 1 and 2. This phenomenon can also be found in complexes {[Cd3(nbta)2(bpa)2]}n13c and {[Cd3(nbta)2(bpy)5(H2O)2](H2O)6}n13d reported by our group. Both complexes possess helical and self-penetrating character.
Complexes 1 and 2 are air stable and retain the crystalline integrity at ambient conditions. Thermogravimetric analysis (see Fig. S8, ESI†) of 1 shows the initial weight loss in the temperature range of 140–210 °C, which can be ascribed to the removal of lattice water molecules (observed: 8.2% and calculated: 7.9%). From ca. 340 °C the expulsion of organic components occurs. The TG curve for 2 indicates that the first weight loss of 3.0% (calculated: 2.9%) corresponding to the loss of two coordinated water molecules. Further weight loss indicates the decomposition of coordination framework.
Weak fluorescence emission bands at ca. 471 and 527 nm for both 1 and 2 are observed (see Fig. S9, ESI†). These emissions are neither metal-to-ligand charge transfer (MLCT) nor ligand-to-metal transfer (LMCT) in nature, since CdII ions are difficult to oxidize or reduce due to their d10 configuration.15 In comparison to that of the free H3nbta, most of the emission maxima of complexes 1 and 2 are significantly shifted (see Fig. S8, ESI†). The shifts of the emission bands are attributed to both the deprotonated effect of H3nbta and the coordination interactions of the organic ligands to CdII ions.16 More detailed theoretical and spectroscopic studies may be necessary for better understanding of the luminescent mechanism.
In conclusion, two new 2D CdII coordination polymers [Cd3(nbta)2(H2O)4] (1) and [Cd3(nbta)2(bbi)2(H2O)2] (2) exhibiting enthralling helical arrays, have been isolated under hydrothermal conditions. Topology analysis shows two unique (4,5,6)-connected networks. A further analysis reveals that both complexes 1 and 2 can be realized as arising from the different linkages of the adjacent 63 nets. The results demonstrate that the 5-nitro-1,2,3-benzenetricarboxylate tectons are good candidates for constructing interesting coordination frameworks. Subsequent works will be focused on syntheses, structures, and physical properties of more coordination polymers with transition/rare metal ions and H3nbta or related derivatives.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21073082, 21071074), 2009GGJS-104 and sponsored by Program for Science & Technology Innovation Talents in Universities of Henan Province (2011HASTIT027).References
- (a) J. P. Zhang, S. L. Zheng, X. C. Huang and X. M. Chen, Angew. Chem., Int. Ed., 2004, 43, 206 CrossRef CAS; (b) S. Hu, J. C. Chen, M. L. Tong, B. Wang, Y. X. Yan and S. R. Batten, Angew. Chem., Int. Ed., 2005, 44, 5471 CrossRef CAS; (c) S. Kitagawa, S. Noro and T. Nakamura, Chem. Commun., 2006, 701 RSC; (d) M. H. Zeng, Q. X. Wang, Y. X. Tan, S. Hu, H. X. Zhao, L. S. Long and M. Kurmoo, J. Am. Chem. Soc., 2010, 132, 2561 CrossRef CAS; (e) M. L. Tong, X. L. Chen and S. R. Batten, J. Am. Chem. Soc., 2003, 125, 16170 CrossRef CAS.
- (a) L. Pan, K. M. Adams, H. E. Hernandez, X. T. Wang, C. Zheng, Y. Hattori and K. Kaneko, J. Am. Chem. Soc., 2003, 125, 3062 CrossRef CAS; (b) S. Y. Yang, L. S. Long, Y. B. Jiang, R. B. Huang and L. S. Zheng, Chem. Mater., 2002, 14, 3229 CrossRef CAS; (c) G. L. Zheng, J. F. Ma, Z. M. Su, L. K. Yan, J. Yang, Y. Y. Li and J. F. Liu, Angew. Chem., Int. Ed., 2004, 43, 2409 CrossRef CAS; (d) M. Du, Z. H. Zhang, L. F. Tang, X. G. Wang, X. J. Zhao and S. R. Batten, Chem.–Eur. J., 2007, 13, 2578 CrossRef CAS; (e) G. P. Yang, Y. Y. Wang, P. Liu, A. Y. Fu, Y. N. Zhang, J. C. Jin and Q. Z. Shi, Cryst. Growth Des., 2010, 10, 1443 CrossRef CAS.
- (a) Q. Fang, G. Zhu, M. Xue, J. Sun, F. Sun and S. Qiu, Inorg. Chem., 2006, 45, 3582 CrossRef CAS; (b) J. Q. Liu, B. Liu, Y. Y. Wang, P. Liu, G. P. Yang, R. T. Liu, Q. Z. Shi and S. R. Batten, Inorg. Chem., 2010, 49, 10422 CrossRef CAS; (c) L. J. Zhou, Y. Y. Wang, C. H. Zhou, C. J. Wang, Q. Z. Shi and S. M. Peng, Cryst. Growth Des., 2007, 7, 300 CrossRef CAS; (d) Z. R. Pan, J. Xu, H. G. Zheng, K. X. Huang, Y. Z. Li, Z. J. Guo and S. R. Batten, Inorg. Chem., 2009, 48, 5772 CrossRef CAS; (e) J. R. Li, Q. Yu, E. C. Sañudo, Y. Tao and X. H. Bu, Chem. Commun., 2007, 2602 RSC.
- (a) G. L. Zheng, J. F. Ma, J. Yang, Y. Y. Li and X. R. Hao, Chem.–Eur. J., 2004, 10, 3761 CrossRef CAS; (b) K. Biradha, M. Sarkar and L. Rajput, Chem. Commun., 2006, 4169 RSC; (c) X. M. Gao, D. S. Li, J. J. Wang, F. Fu, Y. P. Wu, H. M. Hu and J. W. Wang, CrystEngComm, 2008, 10, 479 RSC.
- (a) P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., 1999, 111, 2798 ( Angew. Chem., Int. Ed. , 1999 , 38 , 2639 ) CrossRef; (b) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (c) J. C. Dai, X. T. Wu, Z. Y. Fu, S. M. Hu, W. X. Du, C. P. Cui, L. M. Wu, H. H. Zhang and R. Q. Sun, Chem. Commun., 2002, 12 RSC; (d) G. P. Yang, L. Hou, Y. Y. Wang, Y. N. Zhang, Q. Z. Shi and S. R. Batten, Cryst. Growth Des., 2011, 11, 936 CrossRef CAS.
- (a) T. Y. Niu, X. Q. Wang and A. J. Jacobson, Angew. Chem., 1999, 111, 2059 ( Angew. Chem., Int. Ed. , 1999 , 38 , 1934 ) CrossRef; (b) S. A. Barnett, A. J. Blake, N. R. Champness and C. Wilson, Chem. Commun., 2002, 1640 RSC; (c) D. Li, T. Wu, X. P. Zhou, R. Zhou and X. C. Huang, Angew. Chem., Int. Ed., 2005, 44, 4175 CrossRef CAS; (d) J. R. Li, Y. Tao, Q. Yu and X. H. Bu, Chem. Commun., 2007, 1527 RSC.
- (a) A. F. Wells, Three-dimensional Nets and Polyhedra, Wiley-Interscience, New York, 1977 Search PubMed; (b) A. F. Wells, Further Studies of Three-dimensional Nets, ACA Monograph 8, American Crystallographic Association, p. 1979 Search PubMed.
- (a) C. N. R. Rao, S. Natarajan and R. Vaidhyanathan, Angew. Chem., Int. Ed., 2004, 43, 1466 CrossRef CAS; (b) G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217 CrossRef; (c) Reticular Chemistry Structure Resource (RCSR): http://rcsr.anu.edu.au/..
- (a) R. J. Hill, D. Long, N. R. Champness, P. Hubberstey and M. Schröder, Acc. Chem. Res., 2005, 38, 337 CrossRef; (b) H. Kim, G. Park and K. Kim, CrystEngComm, 2008, 10, 954 RSC.
- (a) R. J. Hill, D. L. Long, M. S. Turvey, A. J. Blake, N. R. Champness, P. Hubberstey, C. Wilson and M. Schröder, Chem. Commun., 2004, 1792 RSC; (b) R. Sun, S. N. Wang, H. Xing, J. F. Bai, Y. Z. Li, Y. Pan and X. Z. You, Inorg. Chem., 2007, 46, 8451 CrossRef CAS.
- (a) O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703 CrossRef CAS; (b) H. Li, C. E. Davis, T. L. Groy, D. G. Kelley and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 2186 CrossRef CAS; (c) H. Li, M. Eddaoudi, T. L. Groy and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 8571 CrossRef CAS; (d) O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS.
- (a) F. Takusagawa and A. Shimada, Bull. Chem. Soc. Jpn., 1973, 46, 2998 CrossRef CAS; (b) F. Mo and E. Adman, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1975, 31, 192 CrossRef; (c) R. Pech and J. Pickardt, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1990, 46, 1928 CrossRef; (d) R. Pech and J. Pickardt, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 1731 CrossRef; (e) S. O. H. Gutschke, D. J. Price, A. K. Powell and P. T. Wood, Angew. Chem., Int. Ed., 2001, 40, 1920 CrossRef CAS; (f) S. H. Dale, M. R. J. Elsegood and A. E. L. Coombs, CrystEngComm, 2004, 6, 328 RSC; (g) S. H. Dale and M. R. J. Elsegood, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2004, 60, 444 CrossRef; (h) F. Guo and K. D. M. Harris, J. Am. Chem. Soc., 2005, 127, 7314 CrossRef CAS; (i) M. Du, Z. H. Zhang and Y. P. You, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2005, 61, O574 Search PubMed.
- (a) L. F. Ma, Q. L. Meng, L. Y. Wang, B. Liu and F. P. Liang, Dalton Trans., 2010, 39, 8210 RSC; (b) L. F. Ma, B. Liu, L. Y. Wang, J. L. Hu and M. Du, CrystEngComm, 2010, 12, 1439 RSC; (c) L. F. Ma, C. P. Li, L. Y. Wang and M. Du, Cryst. Growth Des., 2010, 10, 2641 CrossRef CAS; (d) L. F. Ma, Q. L. Meng, C. P. Li, B. L, L. Y. Wang, M. Du and F. P. Liang, Cryst. Growth Des., 2010, 10, 3036 CrossRef CAS.
- L. F. Ma, L. Y. Wang, M. Du and S. R. Baten, Inorg. Chem., 2010, 49, 365 CrossRef CAS.
- (a) L. L. Wen, D. B. Dang, C. Y. Duan, Y. Z. Li, Z. F. Tian and Q. J. Meng, Inorg. Chem., 2005, 44, 7161 CrossRef CAS; (b) T. L. Hu, R. Q. Zou, J. R. Li and X. H. Bu, Dalton Trans., 2008, 1302 RSC.
- F. F. Li, J. F. Ma, S. Y. Song, J. Yang, Y. Y. Liu and Z. M. Su, Inorg. Chem., 2005, 44, 9374 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional structural figures, TG curves and luminescence spectra of 1 and 2. CCDC reference numbers 806327 and 806328. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ra00119a |
| ‡ Synthesis of 1. A mixture of H3nbta (0.1 mmol, 25.5 mg), Cd(NO3)2·4H2O (0.1 mmol 30.8 mg), KOH (0.1 mmol, 5.6 mg) and H2O 15 mL were placed in a Teflon-lined stainless steel vessel, heated to 160 °C for 3 days, and then cooled to room temperature over 24 h. Colorless block crystals of 1 were obtained. Yield: 46% (based on Cd). Elemental analysis (%): calcd for C18H12Cd3N2O20 C 23.67, H 1.32, N 3.07; found C 23.74, H 1.39, N 3.15. IR (cm−1): 3392m, 1608s, 1560s, 1436s, 1351s, 1112w, 1072m, 927m, 827m, 747m, 713m.Synthesis of 2. 2 was synthesized in the similar way as that described for 1, except that bbi was taken into the reaction. Yield: 34% (based on Cd). Elemental analysis (%): calcd for C38H36Cd3N10O18 C 36.28, H 2.88, N 11.13; found C 36.35, H 2.941, N 11.18. IR (cm−1): 3355m, 1600s, 1523m, 1434s, 1347s, 1106m, 1082m, 941m, 825m, 740s, 717m. |
| § Crystal data for 1: C18H12Cd3N2O20, M = 913.50, monoclinic, space groupP21/c, T = 296(2)K, a = 14.1956(12) Å, b = 13.5254(11) Å, c = 12.3722(10) Å, β = 98.9920(10), V = 2346.3(3) Å3, Z = 4, Dc = 2.586 g cm−3, Reflections collected/unique, 17440/4354 [R(int) = 0.0176], R = 0.0175, and wR = 0.0430 (I > 2σ(I)), R = 0.0199, wR = 0.0442 (all data). |
| ¶ Crystal data for 2: C38H36Cd3N10O18, M = 1257.97, monoclinic, space groupP21/c, T = 296(2)K, a = 11.5392(13) Å, b = 25.502(3) Å, c = 7.6065(8) Å, β = 105.9070(10), V = 2152.7(4) Å3, Z = 2, Dc = 1.941 g cm−3, Reflections collected/unique, 16287/3998 [R(int) = 0.0188], R = 0.0241, and wR = 0.0532 (I > 2σ(I)), R = 0.0279, wR = 0.0548 (all data). |
| This journal is © The Royal Society of Chemistry 2011 |
