Mechanistic investigation of the ring opening metathesis polymerisation of alkoxy and alkyl substituted paracyclophanedienes†
Abstract
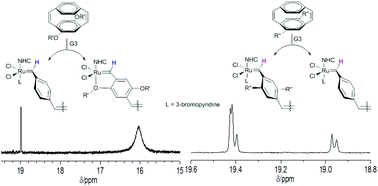
This paper discusses the living nature of the ring opening metathesis polymerisation (ROMP) of alkoxy and alkyl substituted [2.2] paracyclophane-1,9-dienes (M1 and M2), initiated with Grubbs’ second and third generation catalysts (G2 and G3). The active ruthenium carbene chain ends present during the ROMP of these monomers have been identified by in situ1H and 31P NMR spectroscopy and the relative kinetics of the initiation and propagation reactions in the polymerisation determined for both G2 and G3 complexes. The apparent rate constants for initiation of both M1 and M2 using G3 are at least one order of magnitude larger than those determined for polymerisation using G2 and this leads to lower dispersity (Đm) polymers using the G3 catalyst as initiator. Complexation of the ruthenium centre in the active chain end by the oxygen of the alkoxy substituents in M1 is observed in the NMR spectra of the reaction and this leads to significantly slower rates of propagation than those observed for the alkyl derivative M2 using both G2 and G3 complexes. Although the polymerisation requires longer to reach full conversion for monomer M1 the slower propagation generally results in better control of the polymer molecular weight (lower Đm) as the ratio of apparent rate constants for initiation and propagation are larger than those determined for the ROMP of M2.


 Please wait while we load your content...
Please wait while we load your content...