The co-curing process of a benzoxazine-cyanate system and the thermal properties of the copolymers
Xiaodan
Li
and
Yi
Gu
*
College of Polymer Science and Engineering, State Key Laboratory of Polymeric Materials Engineering, Sichuan University, Chengdu, Sichuan 610065, P. R. China. E-mail: guyi@scu.edu.cn; Fax: +086-28-85405138; Tel: +086-28-85400377
First published on 19th October 2011
Abstract
Investigations confirmed the co-reactions between oxazine rings and triazine rings formed a crosslinking network composed of triazine rings as part of the polybenzoxazine matrix. The copolymers have higher initial storage modulus compared to polycyanate ester and exhibit better thermal stabilities than the polybenzoxazine.
Introduction
Polybenzoxazine is a class of high performance polymer possessing superior mechanical properties, high temperature stability and high glass transition temperature (Tg).1,2 The polymers are characterized by –CH2–NR–CH2– Mannich bridges and undergo polymerizationvia thermally induced ring-opening in the course of which no volatiles formed. As traditional thermosetting resins, it is hard for benzoxazine resins to meet the requirements of molding technology and comprehensive performance simultaneously. Therefore, in addition to the development and preparation of novel benzoxazine monomers, effort has also been focused on the introduction of different segments into the crosslinking network of the benzoxazine resins by blending, copolymerization, increasing the appropriate point in the crosslinking system and forming a certain phase structure to modify the benzoxazine.3–7Cyanate ester is a well-known thermosetting resin with good thermal properties, low dielectric loss and high Tg.8–11 The cyanate ester monomer undergoes thermal self-cyclotrimerization to form a three-dimensional network structure of polycyanurate.12Bisphenol A dicyanate ester (BACY) is a common cyanate ester monomer with wide applications.13 Introduction of cyanate ester with high Tg and toughness into a polybenzoxazine matrix should result in a new matrix with some salient features of the former. But there is little research on the co-curing reaction of the two resins, only preliminary studies by Kumar et al.14 So, obviously, in order to get better performance, further studies on the various interactions in the system and the structures of the copolymer are still necessary.
In this paper, the thermosetting blends composed of diamine-based benzoxazine (P-ddm) and bisphenol A dicyanate ester (BACY) were prepared. The co-curing process of the blends, the structures and thermal properties of the copolymers were investigated by differential scanning calorimetry (DSC), Fourier transform-infrared spectroscopy (FT-IR) and dynamic mechanical analysis (DMA).
Experimental
Materials
Diamine-based benzoxazine monomer (P-ddm) was synthesized from phenol, 4,4′-diaminodiphenyl methane and paraformaldehydeviaMannich reaction. Bisphenol A dicyanate ester (BACY) was purchased from the Research Institute for Special Structures of Aeronautical Composites AVIC and was used without further purification.Preparation of blends and copolymers
The thermosetting blends of P-ddm and BACY at different stoichiometries were prepared by melting. The samples for DMA experiment were obtained by curing the molten resin in a rectangular mold. According to the DSC results, the curing schedules of the blend, P-ddm and BACY were determined as following: (1) blend, 120 °C/1 h, 140 °C/2 h, 180 °C/2 h, 210 °C/2 h and 230 °C/2 h, (2) P-ddm, 120 °C/1 h, 140 °C/2 h, 160 °C/2 h, 180 °C/2 h and 210 °C/2 h, and (3) BACY, 120 °C/1 h, 140 °C/2 h, 180 °C/2 h, 210 °C/2 h, 230 °C/2 h and 250 °C/2 h.Experimental characterization
FT-IR spectra were recorded with a Nicolet Magna 650 spectrophotometer at a resolution of 4 cm−1. DSC thermograms were obtained from a TA instrument model Q20 at a heating rate of 10 °C min−1 under nitrogen. DMA tests were performed on a TA instrument model Q800 at a frequency of 1.0 HZ and a heating rate of 5 °C min−1 in bending mode. The size of the DMA samples was 30 × 10 × 3 mm.Results and discussion
DSC analysis for co-curing reaction of the blend
On thermal curing, P-ddm undergoes ring-opening polymerization to form polybenzoxazine. BACY is polymerized by a cyclotrimerization of the cyano groups and a very high temperature, beyond 300 °C, is usually required without catalyst. The DSC thermograms of P-ddm and BACY are given in Fig. 1 for comparison. However, different from the only one exothermic peak at 240 °C or 331 °C in the DSC curves of each monomer, there were four distinct wider exothermic peaks at 180 °C, 227 °C, 240 °C and 287 °C, named A, B, C and D, observed in the curves of the blend. The curing enthalpy values of the P-ddm and the BACY are 139 kJ mol−1 and 189 kJ mol−1, respectively. It means that if there were only the self-polymerizations of the two monomers, the theoretic enthalpy value of the blend would be 164 kJ mol−1. However, the actual one is 199 kJ mol−1 from all four exothermic peaks collectively, which implies more reactions occurred in the blend. Simultaneously, compared to each component, the blend has better processability with lower curing onset temperature and wider exothermic peak.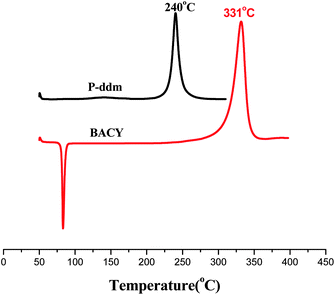 | ||
| Fig. 1 DSC thermograms of P-ddm and BACY. | ||
Obviously, the peak C in Fig. 2 is due to the self-polymerization of P-ddm at 240 °C. But the exothermic peak of BACY at 331 °C disappeared while three new peaks, A, B and D were observed. It is clear that the self-polymerization of BACY will be affected by P-ddm. According to the literature,15 the curing of cyanate ester can be catalyzed by a small amount of hydroxyl groups. Therefore, the peak A is due to the polymerization of BACY catalyzed by the residual phenolic hydroxyl groups in the benzoxazine monomers while the peak B is also caused by the phenolic hydroxyl groups but from the ring-opening of P-ddm at 140 °C (Scheme 1a). At 180 °C, with the disappearance of the peaks A and B, the cyano groups had been exhausted. Because of the self-polymerization of P-ddm (Scheme 1b), the peak C decreased greatly after 210 °C. Finally, there was no exothermic peak indicating the blend had cured completely at 230 °C.
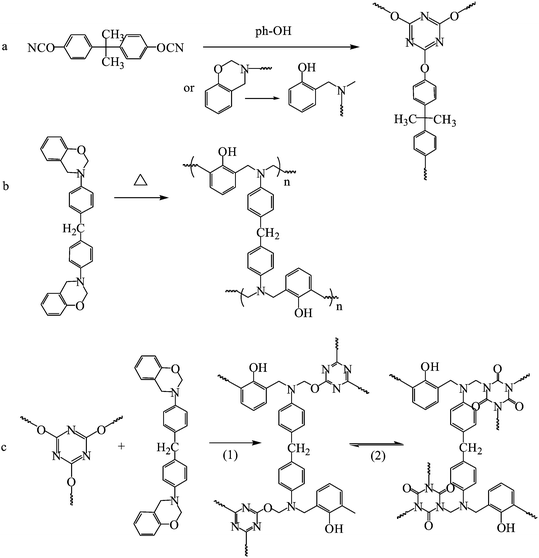 | ||
| Scheme 1 (a) The polymerization of BACY with catalysts. (b) The self-polymerization of P-ddm. (c) The reaction between P-ddm and the triazine ring: (1) the insertion of P-ddm into the triazine ring and (2) the isomerization of the generated cyanurate. | ||
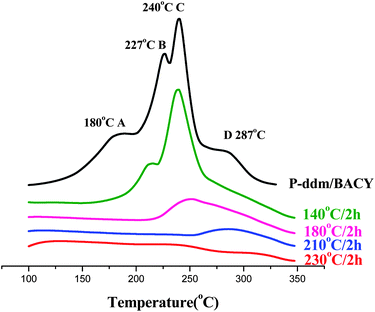 | ||
| Fig. 2 DSC thermograms of the blends cured at different temperatures. | ||
FT-IR analysis for co-curing reaction
FT-IR was used to characterize the changes of different functional groups. FT-IR spectra of the samples from different curing stages are shown in Fig. 3 and the significant peaks of groups are given below: ν 947 cm−1 (oxazine ring), ν 2237 cm−1 (cyanate), ν 1368 cm−1 (triazine ring), ν 1680 cm−1 (isocyanurate).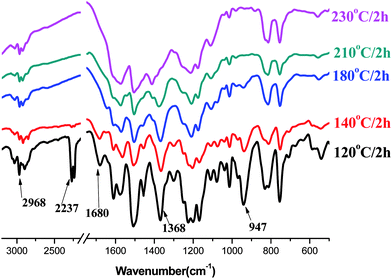 | ||
| Fig. 3 FT-IR spectra of the blends cured at different temperatures. | ||
In the FT-IR spectra, there was a significant peak assigned to the characteristic absorption of triazine ring at 1368 cm−1 due to the residual hydroxyl groups in the system catalyzing the formation of the triazine rings at lower temperature. The oxazine rings are opened with the increase in temperature. Firstly, the generated phenolic hydroxyl groups further catalyze the cyclotrimerization of the cyano groups leading to the first increase of the peak at 1368 cm−1. And then, the opened oxazine rings can insert into the triazine rings to form cyanurates some of which will isomerize to isocyanurates with the observation of the characteristic absorption of the isocyanuric groups at 1680 cm−1 and the final decrease of the peak assigned to the cyanurates at 1368 cm−1 (Scheme 1c). At 180 °C, according to the co-curing process of cyanate ester and epoxy resin,16 the generated isocyanurates probably further react with oxazine rings to form other structures with the disappearance of the peak at 1680 cm−1. After 210 °C, the peak at 947 cm−1 disappeared because of the complete polymerization of the oxazine rings. And there was almost no change in the FT-IR spectra at 230 °C with the final formation of a stable crosslinking network composed of triazine rings as part of the polybenzoxazine matrix.
DMA of the copolymers
The changes in the polymer structures will affect the thermal performance. The DMA diagrams of the copolymers at different stoichiometries are shown in Fig. 4. With the increase of P-ddm, the hydrogen bonds in the polybenzoxazine matrix improved the initial storage modulus of the copolymers. Actually, the hydrogen bond is a weak physical interaction which will be destroyed by the movements of the molecular chains with a rapid decline of modulus when the temperature is above the Tg. But the formation of more rigid and stable triazine rings in the crosslinking network will increase the Tgs and the retentions of the storage modulus of the copolymers at high temperature. In conclusion, the copolymers have a higher initial storage modulus compared to polycyanate ester and exhibit better thermal stabilities at high temperature than polybenzoxazine.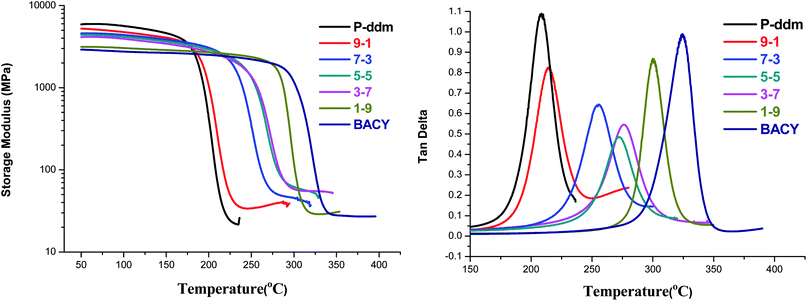 | ||
Fig. 4 DMA diagrams of different polymeric systems; P-ddm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BACY = 9 BACY = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7 1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5 3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3 5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 1 7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (from top to bottom). 9 (from top to bottom). | ||
Conclusion
The curing process and thermal properties of the novel blends composed of P-ddm and BACY were studied. There were four exothermic peaks in the DSC curve of the blend. Compared to each component, the blend have better processability. The FT-IR analysis shows the co-reactions between oxazine rings and triazine rings will lead to form a crosslinking network composed of triazine rings as part of the polybenzoxazine matrix. So, the copolymers have higher initial storage modulus compared to polycyanate ester and exhibit better thermal stabilities than the polybenzoxazine with higher Tgs and retentions of the storage modulus at high temperature.Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (Project No. 50873062).Notes and references
- X. Ning and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 1121 CrossRef CAS.
- S. Rimdusit and H. Ishida, Polymer, 2000, 41, 7941 CrossRef CAS.
- Y. Liu and C. Chou, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5267 CrossRef CAS.
- T. Takeichi, T. Agag and R. Zeidam, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 2633 CrossRef CAS.
- B. Kiskan and Y. Yagci, Polymer, 2005, 46, 11690 CrossRef CAS.
- Q. Chen, R. Xu, J. Zhang and D. Yu, Macromol. Rapid Commun., 2005, 26, 1878 CrossRef CAS.
- Y. Cui, Y. Chen, X. Wang, G. Tian and X. Tang, Polym. Int., 2003, 52, 1246 CrossRef CAS.
- H. Ishida and D. Allen, Polymer, 1996, 37, 4487 CrossRef CAS.
- M. S. Lakshmi and B. S. R. Reddy, Eur. Polym. J., 2002, 38, 795 CrossRef.
- G. Liang and M. Zhang, J. Appl. Polym. Sci., 2002, 85, 2377 CrossRef CAS.
- M. Laskoski, D. D. Dominguez and T. M. Keller, J. Mater. Chem., 2005, 15, 1611 RSC.
- C. P. R. Nair, D. Mathew and K. N. Ninan, Adv. Polym. Sci., 2001, 155, 1 CrossRef CAS.
- G. Liang and M. Zhang, J. Appl. Polym. Sci., 2002, 85, 2377 CrossRef CAS.
- K. S. S. Kumar, C. P. R. Nair and K. N. Ninan, Eur. Polym. J., 2009, 45, 494 CrossRef CAS.
- C. H. Lin, S. L. Chang, C. W. Hsieh and H. H. Lee, Polymer, 2008, 49, 1220 CrossRef CAS.
- K. Ravi Sekhar, Kishore and S. Sankaran, J. Appl. Polym. Sci., 2008, 109, 2023 CrossRef.
| This journal is © The Royal Society of Chemistry 2011 |
