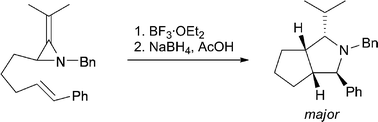
2-Methyleneaziridines can be tethered to a variety of alkene or alkyne acceptors through the saturated carbon of the heterocyclic ring by application of a simple lithiation/alkylation sequence (8 examples, 31–59%). Treatment of the resultant alkene bearing substrates with BF3·OEt2 leads to cis-octahydrocyclopenta[c]pyrroles in which up to four contiguous stereocentres are created in a diastereocontrolled manner after reductive work-up. Using an alkyne based substrate, a 2,4,5,6-tetrahydrocyclopenta[c]pyrrole is produced by rapid tautomerisation of the initially formed bisenamine. Evidence that these (3 + 2) ‘cycloadditions’ proceed in a stepwise manner via a 2-aminoallyl cation is presented.