François-Moana Gautier, Simon Jones, Xianfu Li and Stephen J. Martin
Org. Biomol. Chem., 2011,9, 7860-7868
DOI:
10.1039/C1OB05965C,
Paper
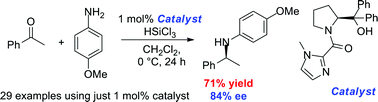
A highly active organocatalyst has been shown to affect the asymmetric reductive amination of