Highly-controlled regiospecific free-radical copolymerization of 1,3-diene monomers with sulfur dioxide†
Abstract
The free-
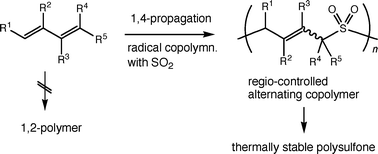
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...