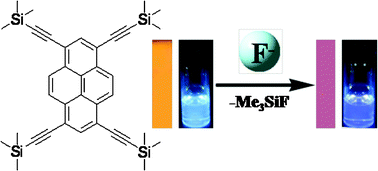
Pyrene derivative 1 containing four trimethylsilylethynyl substituents was synthesized and investigated as a chromogenic and fluorescent chemodosimeter sensor for fluoride ions. 1 showed a high sensitivity and specific selectivity over a rapid response time toward fluoride anions compared to other anions, such as Cl−, Br−, ClO4−, H2PO4− and HPO42−. TD-DFT calculations showed that the delocalization of the σ-electrons of the silicon destabilized the HOMO energy level of 1, thus red shifting both its absorption and emission spectrum. The addition of F− removed the trimethylsilyl substituents and resulted in a blue shift of both the absorption and fluorescent spectra of 1, which could be monitored by the color change with the naked-eye. Moreover, an easy to prepare test paper, which was obtained by immersing a filter paper into a THF solution of 1, could be utilized to detect and estimate the concentration of fluoride anions in water.