Free radical 5-exo-dig cyclization as the key step in the synthesis of bis-butyrolactone natural products: experimental and theoretical studies†‡
Abstract
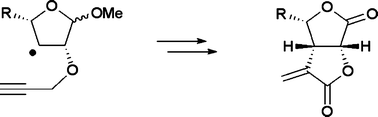
Radical cyclization reactions were performed by 5-exo-dig mode to yield cis-fused bicyclic systems, leading to the synthesis of bis-butyrolactone class of natural products. The study was aimed at understanding the impact of
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...