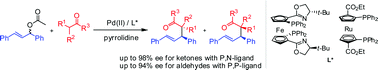
Palladium-catalyzed asymmetric allylic alkylation of ketones, via enamines generated in situ as nucleophiles, were carried out smoothly with chiral metallocene-based P,N-ligands. Under the same conditions, however, reactions of aldehydes could hardly be observed. Subsequently, this obstacle was resolved by using chiral metallocene-based P,P-ligands. Both ketones and aldehydes afforded excellent enantioselectivities with up to 98% ee and 94% ee, respectively.