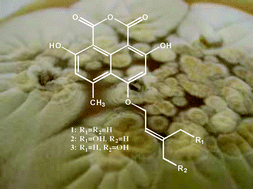
The marine-derived fungus Coniothyrium cereale was isolated from the green alga Enteromorpha sp. and found to produce the new phenalenone derivatives 1–7 as well as the known compounds lactone 8, (−) sclerodin (9), lamellicolic anhydride (10), (−) scleroderolide (11), and (−) sclerodione (12). The structures of these closely related compounds were established from extensive spectroscopic investigations on the basis of one and two dimensional NMR spectroscopic studies (1H, 13C, COSY, NOESY, HSQC and HMBC) as well as mass spectrometric analysis (LC/MS, HREIMS and HRESIMS), UV and IR spectra. Compounds 5 and 11 showed the same antimicrobial activity toward Staphylococcus aureus SG 511 with an MIC value of 24 μM. The presence of a diketo-lactone ring as in compounds 5 and 11 was found to be essential for this activity. In agar diffusion assays with Mycobacterium phlei considerable inhibition zones were observed for compounds 2, 4 and 7. Compounds 1, 5 and 9 showed potent inhibition of human leukocyte elastase (HLE) with IC50 values of 7.2, 13.3 and 10.9 μM, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...